
Endoscopic mucosal resection and full-thickness resection of neoplastic lesions of the colon: clinical application and techniques—a narrative review
Introduction
Background
Colorectal cancer (CRC) is the fourth most common cancer worldwide and the third leading cause of cancer related death (1). Colonoscopy with polypectomy is an effective modality to prevent CRC with an estimated 60–90% reduction of CRC incidence (2,3). Most colorectal polyps can be effectively resected using snare polypectomy and biopsy forceps. However, nearly 10–15% of polyps are classified as “difficult” due to their location, size, or morphology (4). These lesions were traditionally managed surgically with significant potential morbidity and mortality. Advances in endoscopic techniques and tools have allowed safe and effective endoscopic removal of difficult colorectal lesions potentially avoiding surgical intervention.
Rationale and knowledge gap
Endoscopic mucosal resection (EMR) was pioneered in Japan in 2003 for the management of early gastric cancer (5). The indications have extended to various gastrointestinal lesions including colorectal polyps, and has currently gained acceptance as the first line therapy for most large colorectal lesions. Several variations of EMR have been described since including underwater EMR (UEMR) and pre-cut EMR. Piecemeal resection is often required during EMR leading to inability to identify margin status on pathologic review. As opposed to EMR, endoscopic submucosal dissection (ESD) allows en-bloc resection of lesions using special electrosurgical knives overcoming the limitations of EMR. The higher en-bloc resection and accurate pathologic assessment associated with ESD come at the expense of higher technical challenges and in some series, increased procedural complication rates (6). Endoscopic full-thickness resection (EFTR) is the latest addition to the endoscopic resection techniques designed to overcome certain limitations of EMR and ESD. It allows complete transmural resection of difficult colorectal lesions including recurrence with scar formation after previous endoscopic resection and subepithelial lesions extending beyond the submucosa. Selecting the appropriate resection strategy for each colorectal lesion remains challenging as data comparing different resection techniques continues to emerge.
Objective
In this paper, we aim to describe the technical aspects and limitations of EMR and EFTR for colorectal lesions, highlighting recent advances and technical challenges, for the practicing endoscopist to be able to understand the role of these techniques and therefore identify the appropriate resection strategy for colorectal lesions. We present this article in accordance with the Narrative Review reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-24-10/rc).
Methods
We searched original research papers, literature reviews, and meta-analysis available on PubMed up until February 2024 discussing EMR and EFTR in the resection of colorectal lesions. We used the following terms: “Endoscopic mucosal resection” AND “colorectal polyps”, “Endoscopic full-thickness resection” AND “colorectal polyps”. Meta-analysis and randomized trials were predominantly used to compare outcomes between different endoscopic resection techniques. The summary of the search strategy is provided in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | February 1st, 2024 |
| Databases searched | PubMed |
| Search terms used | “Endoscopic mucosal resection” AND “colorectal polyps” “Endoscopic full-thickness resection” AND “colorectal polyps” |
| Timeframe | 1955–2024 |
| Inclusion and exclusion criteria | Full-text articles involving adult patients who underwent endoscopic mucosal resections or endoscopic full-thickness resection for lesions involving the colorectum were reviewed. This included original research papers, literature reviews, and meta-analysis. Only publications written in English were reviewed |
| Publications discussing endoscopic resection of lesions in the upper gastrointestinal tract were excluded | |
| Selection process | Literature search was conducted independently by F.N. and P.S.G. Both authors discussed the literature selection and reached a consensus |
Technical considerations for endoscopic mucosal resection
EMR is a standard variation of standard polypectomy using specialized devices with the ability to remove lesions confined to the superficial layers (mucosa and submucosa) of the gastrointestinal tract. The commonly used techniques are categorized under injection-, cap-, ligation-assisted, and UEMR. In this review, we will focus on injection-assisted and UEMR, that are commonly used for the resection of colorectal neoplastic lesions (7).
First described in 1955 for rigid sigmoidoscopy (8) and 1973 for flexible colonoscopy (9), injection-assisted EMR consists of injecting a solution into the submucosal space under the lesion to create a safety cushion (Figure 1). Submucosal injection is performed using a retractable injection needle through the working channel of the endoscope. For large lesions, multiple injections may be needed to achieve an adequate lift and should be targeted to optimize visualization and resection. A static or dynamic submucosal injection technique may be used. A dynamic technique consists of redirecting the needle position during submucosal injection allowing rapid and adequate submucosal expansion (10).

Various solutions have been evaluated and used for injection. While initial descriptions of EMR used normal saline as the lifting agent, subsequent solutions containing hyaluronic acid have been demonstrated to sustain mucosal lifting longer than saline solutions and therefore reducing the volume of solution necessary to complete an EMR (11). Further studies evaluating hydroxypropyl methylcellulose, succinylated gelatin, glycerol, and fibrinogen solutions demonstrated similar results (12-14). However, a meta-analysis comparing several injection solutions showed no clear advantage for any single substance in terms of complete resection rate, although there was a favorable trend for succinylated gelatin, hydroxyethyl starch, and fibrinogen mixture to reduce post-polypectomy bleeding compared with saline and 50% dextrose water (15). To note, ORISE Gel lifting agent (Boston Scientific, Marlborough, MA, USA), initially Food and Drug Administration (FDA) approved in 2018, was associated with a multi-nucleated giant cell reaction leading to submucosal distortion which could potentially be mistaken for submucosal tumor growth. The gel was subsequently retracted from the market in December 2022 (16).
Staining dye such as indigo carmine and methylene blue is typically added to the injection solution in order to better delineate the deep and lateral margins of the lesion and may help in early recognition of muscularis propria injuries and perforation. If the muscularis propria is inadvertently resected during EMR, the transected surface will not be stained leading to a target sign and effective recognition of deep mural injury (17).
In addition, dilute epinephrine (1:100,000–1:200,000) is sometimes added to maintain a clean resection field and reduce procedural bleeding (18,19). A meta-analysis including 1,388 patients demonstrated that epinephrine injection significantly reduced overall and early post-polypectomy bleeding but did not affect delayed bleeding risk (20). In addition, epinephrine may theoretically reduce vascular flow and delay fluid reabsorption therefore enhancing the duration of the submucosal cushion.
Both hot-snare and cold-snare EMR are variations of injection-assisted EMR. In hot-snare EMR, electrosurgical cautery is used, wherein electrical current converted into heat transects the tissue when the snare is closed. In cold-snare EMR, the polyp is removed without electrocautery after submucosal injection (Figure 2).

Both cold-snare and hot-snare EMR have high rates of complete histologic and macroscopic resection (21,22). Regarding adverse events, both techniques have similar rates of intraprocedural bleeding; however, for large sessile serrated lesions (SSLs), delayed bleeding was lower with cold-snare EMR (22,23). Similarly, a meta-analysis including 911 patients who underwent resection of 1,137 SSLs found lower rates of delayed bleeding (0% vs. 2.3%, P=0.03) and residual polyp rate (0.9% vs. 5%, P=0.01) with cold EMR compared to hot EMR (24). Cold-snare EMR may be an ideal technique for SSLs due to their thin mucosal layer and little to no submucosal fibrosis. For large adenomatous lesions, cold-snare EMR has been associated with higher recurrence rate on follow-up examination, although recurrences can be effectively treated (25).
In UEMR, no submucosal injection is needed. Air is suctioned and water is instilled to immerse the target lesion, thereby ‘floating’ the mucosa and submucosa away from the muscularis propria. Electrocautery is typically used, although resection can be performed with cold snare. This technique has the potential advantage of allowing easier resection of flat lesions. In addition, it was shown to be effective in managing recurrences after EMR and fibrotic lesions that may not be adequately lifted (26,27). Comparative studies have demonstrated the advantages of UEMR over hot-snare EMR. A meta-analysis found higher complete macroscopic resection rate with UEMR compared to hot-snare EMR, lower rates of recurrence on follow-up, and similar rates of immediate or delayed bleeding and perforation (28).
Several variations of injection-assisted EMR have been recently described. In pre-cut EMR, after submucosal injection, an electrosurgical knife or snare tip is used for full or partial circumferential incision before snare resection (Figure 3). This technique is particularly useful for lesions 20–30 mm in size where en-bloc resection is needed, or non-lifting lesions <20 mm (29). A recent meta-analysis demonstrated significantly higher en-bloc resection with fewer recurrence events than EMR, and faster procedure times than ESD, with similar en-bloc resection and adverse events (30). Tip-in EMR requires the use of the snare tip to make a mucosal incision at the proximal side of the lesion, which helps fix the snare tip in the submucosal layer, allowing endoscopists to maneuver the snare in the appropriate position (Figure 4). A randomized controlled trial showed significantly higher rates of en-bloc resection (92.4% vs. 76.4%, P=0.005) and complete resection (89.5% vs. 74.5%, P=0.01) for 10–25 mm colorectal polyps with tip-in EMR compared to conventional EMR (31).


Adverse events following colorectal EMR include bleeding and perforation with bleeding being the most common (Figure 5). Intraprocedural bleeding after colorectal EMR is reported between 11% and 22% (32). Multiple tools can be effectively used to achieve hemostasis such as hemostatic forceps, bicap probes, argon plasma coagulation (APC), endoscopic clips, and soft coagulation with the tip of the snare (33). Risk factors include larger lesion size, Paris classification 0–IIa + Is, tubulovillous or villous histology, and centers with low procedural volume (32). Clinically significant delayed bleeding was reported at 6% in a large prospective multicenter study requiring endoscopic therapy in 34% of the cases. Risk factors include proximal colonic location, intraprocedural bleeding, and size of the polyp (32). Perforation is rare after EMR (<1%) and can be identified by careful examination of the resection defect for a ‘target’ sign. Small perforation recognized during the procedure can be effectively treated with endoscopic clips (34,35).

EFTR: technical considerations, efficacy, and safety
Endoscopic resection of colorectal lesions has traditionally been limited to the mucosa and submucosa while leaving the muscularis propria intact to preserve the integrity of the bowel wall. The advancements in natural orifice transluminal endoscopic surgery (NOTES) and introduction of the over-the-scope clip (OTSC) paved the way for transmural endoscopic interventions (36).
There are two approaches for EFTR, exposed and non-exposed EFTR. In exposed EFTR, full-thickness resection is initially performed exposing the peritoneum followed by closure of the defect (Figure 6). Non-exposed EFTR is the most commonly used approach in Western countries and involves serosa-to-serosa apposition followed by full-thickness resection with the assistance of a dedicated full-thickness device (Ovesco Endoscopy, AG, Tubingen, Germany) (Figure 7). The full-thickness resection device (FTRD) consists of an applicator cap mounted on the colonoscope with an OTSC at the tip of the cap and a snare running alongside the scope. A hand wheel mounted on the scope is used to deploy the OTSC. Its appeal over other strategies includes the ability to perform resection and closure with a single device without exposing the peritoneal cavity. The technique is as follows. The lesion is marked circumferentially using a marking probe. A test cap (prOVE Cap, Ovesco Endoscopy) can initially be used to ensure the endoscope will be able to reach and grasp the target lesion. Subsequently, The FTRD is then advanced to the marked lesion. Grasping forceps are used to pull the lesion into the cap while avoiding suction to minimize the risk of adjacent organ entrapment. Once the lesion is fully retracted into the cap and all markings are visible inside the cap, the clip is deployed followed by immediate closure of the snare and cautery is applied to resect the lesion 2 mm above the clip. The lesion is removed, and the resection site is examined to assess for completeness of resection and for any adverse events.

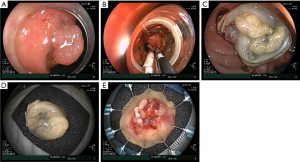
Early preclinical data using the FTRD in an animal model demonstrated safety and efficacy (37). In 2014, the first clinical cases using the dedicated FTRD were reported (38,39). The first retrospective series included 24 patients at two European centers. Technical success was achieved in 83.3% and R0 resection in 75% of patients. Two patients developed post-polypectomy syndrome without other reported adverse events (40). Subsequently, multiple studies have been published and demonstrated excellent technical and clinical success (39). One of the largest prospective multicenter studies to date included 181 patients recruited for EFTR with the FTRD system. Indications included difficult adenomas (n=143), early carcinomas (n=15), and subepithelial tumors (n=23). Technical success was achieved in 89.5% and R0 resection in 76.9% of cases. Adverse events were noted in 9.9% of the cases and included perforation (6/18), bleeding (4/18), post-polypectomy syndrome (3/18), acute appendicitis (3/18), abdominal pain (1/18), and enterocolonic fistula (1/18). Emergency surgery was required in 2.2% of patients (41).
Studies have shown slight variations of outcomes of FTRD depending on the indication. The Dutch colorectal EFTR prospective registry including 367 EFTR procedures from 20 hospitals reported a technical success rate of 83.9% with an R0 resection rate of 82.4%. Adverse events were noted in 9.3% of the cases with 2.7% requiring emergency surgery. Recurrence or residual lesions was only 6.4%. To note, the Dutch registry included a high proportion of patients with T1 carcinoma (n=221) undergoing EFTR (42).
EFTR has also been used as an effective tool for T1 CRC as it provides accurate histological risk assessment. In a retrospective study evaluating the value of EFTR in 156 patients with early CRC, 62% were classified low-risk based on histological features and surgery was not deemed necessary (43). A recent analysis of 330 cases of T1 CRC in the Dutch registry, curative resection rate was 60.8% after excluding deep submucosal invasion (SMI) as a risk factor (44). In a US academic center, the use of FTRD in 48 patients with incompletely resected malignant colorectal lesions resulted in 90.9% R0 resection rate and 79.5% curative resection. Only two patients underwent formal surgical resection for residual pT1 adenocarcinoma with high-risk features (45).
For lesions involving the appendiceal orifice, a recent study including 66 patients demonstrated 89% technical success rate and R0 resection rate of 79% using the FTRD system. However, 17.2% developed appendicitis and 10.3% required appendectomy. Risk factors included male sex and failure to achieve histologic full-thickness resection (46).
There is limited data regarding FTRD for colorectal subepithelial lesions. However, a study of 40 patients with rectal neuroendocrine tumors reported a technical success rate of 100%, R0 resection rate of 100% and no adverse events (47). A subgroup analysis of 80 colorectal subepithelial lesions from the German FTRD registry also demonstrated excellent outcomes with 97.3% technical success rate and 97% R0 resection rate (48). Overall, FTRD of subepithelial colorectal lesions appears to be very effective with improved technical success compared to other indications.
In a systematic review evaluating EFTR for colorectal lesions in 1,936 patients which included the German FTRD registry of 1,178 cases (48), technical success was 86.5%, R0 resection rate was 78.4%, and adverse event rate 15.4%. The most common adverse event was gastrointestinal hemorrhage at 6.4%, followed by perforation at 4.4%, and 1.7% had post-polypectomy syndrome. Overall, 5.5% of patients required surgery. The rate of acute appendicitis following EFTR of appendiceal lesions was 11.5%. The overall rate of residual lesion was 8.3% (49).
Identifying the appropriate resection strategy
Proper patient selection and lesion assessment is a critical first step to determine the appropriate resection strategy. The selection of the appropriate modality depends on the morphology, location, histology, and presence of fibrosis. EMR remains the standard of care in Western countries for the resection of most lesions 10 to 20 mm in size with reasonable safety and efficacy (50). For larger lesions, selection of the appropriate resection technique is challenging. This is due to improved EMR outcomes, the expanding toolkit for endoscopic resection, limited expertise in ESD and EFTR, and resource allocation. Selecting the optimal therapy mainly relies on making an accurate initial diagnosis. Image enhanced endoscopy techniques such as narrow-band imaging (NBI) are commonly used to distinguish benign lesions, adenomas, and adenocarcinomas. The NBI International Colorectal Endoscopic (NICE) and Japan NBI Expert Team (JNET) can discriminate adenomas from adenocarcinomas with a 70–90% accuracy (51). In addition, it is important to differentiate between superficial (<1,000 µm) and deep SMI (≥1,000 µm). CRC with SMI carries a 7–14% risk of lymph node metastasis (52,53). Patients with deep SMI should undergo radical colectomy given the risk for nodal metastasis. Endoscopic evidence of NICE type III pattern on NBI evaluation, Kudo class V/Vn pit pattern, or Paris III lesion is consistent with deep SMI and these patients should be referred for surgical resection. However, current evidence supports that cancer with superficial SMI without lymphovascular invasion, grade 2/3 tumor budding, or poorly differentiated component have no risk of lymph node metastasis and could be effectively treated endoscopically (54).
EMR is the technique of choice for most colorectal polyps larger than 10 mm as it is highly effective, broadly available, relatively safe, and less morbid than the surgical alternative. Although many factors were previously considered a limitation for performing EMR, the expanding endoscopic toolkit and improved EMR outcomes made many of these lesions amenable to EMR. As such, modified EMR techniques have been proposed to resect polyps with severe submucosal fibrosis. When tip-in EMR was compared to ESD for 20 to 30 mm laterally spreading lesions (LSLs) including fibrotic lesions, ESD had a higher en-bloc resection (99% vs. 85%, P<0.001) and R0 resection rates (91% vs. 63%, P<0.001); however, tip-in EMR had a significantly shorter procedure time (8 vs. 60 min, P<0.001) and similar local recurrence rate (2% vs. 0%, P=0.386) (55). Therefore, tip-in EMR may be an effective alternative to ESD in 20 to 30 mm nonpedunculated polyps. Similarly, UEMR has also been associated with a higher R0 resection and complete removal rate of recurrences compared to conventional EMR, and may be effective when conventional EMR fails (26,56). Hot-forceps avulsion and cold-forceps avulsion have an acceptable safety profile and can be used to ablate resistant polyp islands during colorectal EMR (57,58).
High local recurrence rate has been a concern for piecemeal EMR for colorectal lesions larger than 2 cm in size. When piecemeal EMR is performed, recurrence rate is reported around 20% (59-61). However, subsequent endoscopic therapy of recurrences is successful in 91.4% of the cases. In a series of 1,000 patients undergoing EMR of LSLs ≥20 mm in size, early recurrent/residual adenoma at 4 months occurred in 16% of the cases, of which 93.1% were successfully managed endoscopically (62). In a large prospective cohort including 213 patients with residual or recurrent adenoma following EMR of large non-pedunculated polyps, endoscopic therapy of recurrence was successful in 92.5% (63). US Multi-Society Task Force (USMSTF) recommends patients with polyps resected in piecemeal EMR undergo their first surveillance colonoscopy at 6 months, second surveillance colonoscopy at 1 year from their first surveillance, and third at 3 years from the second surveillance, therefore mitigating the risk of recurrence by allowing early detection and effective therapy (64). In addition, several methods have been used to decrease recurrence rate including cauterization of the polyp resection margin with APC, snare tip soft coagulation (STSC), and circumferential removal of normal mucosa adjacent to the resection margin leading to more than 50% reduction in adenoma recurrence rate and further improving the outcomes of piecemeal EMR (65). In a randomized trial comparing thermal therapy of the defect margin by STSC or APC for large colorectal polyps, there was no statistically significant difference in recurrence rates (4.6% with STSC, 9.3% with APC, and 21.4% for controls) and STSC was faster to apply (66).
However, the inability to identify margin status on pathological review remains the major limitation of piecemeal EMR. This is particularly important in lesions with superficial submucosal invasive cancer where adequate en-bloc resection could lead to a potential cure and avert the need for subsequent surgical resection. ESD has specific advantages over EMR, with higher en-bloc resection and lower recurrence rates due to the limitations of EMR for large colorectal lesions. In addition, ESD allows adequate histopathological diagnosis regarding the depth of invasion and lateral margins, without risking to fragment the carcinomatous part (67). In a large multicenter prospective trial of 1,814 patients undergoing ESD of colorectal lesions larger than 20 mm, none of the patients with curative resection had metastatic disease at 5 years, and local recurrence was only 0.5%, all of which were treated endoscopically (68). Nevertheless, colorectal ESD remains technically challenging, time consuming, with a steep learning curve.
Gross morphology and location have been shown to predict the risk for SMI and subsequently the need for en-bloc resection. LSLs are lesions that extend laterally larger than 1 cm. Non-granular LSLs have an estimated 39% risk of SMI compared to 19% for granular LSLs. The invasion site for granular LSLs was the large nodule in 56%, the depressed area in 28% and multifocal in 16%. In comparison, SMI was multifocal in 45% of non-granular LSLs (69). In a meta-analysis including 48 studies, the overall risk of SMI in LSLs was 8.5%. The risk differed among LSL subtypes, with the highest risk in non-granular pseudodepressed LSLs at 31.6%, followed by 10.5% in granular nodular mixed LSLs, 4.9% in non-granular flat elevated LSLs, and only 0.5% in granular homogeneous LSLs. SMI was also more common distally than proximally and increased with lesion size (10–19 mm, 4.6%; 20–29 mm, 9.2%; ≥30 mm, 16.5%) (70). Due to the substantial risk of multifocal SMI, ESD is recommended for non-granular LSLs, and en-bloc resection for large granular LSL nodules, whereas homogeneous granular LSLs especially in the proximal colon are appropriate for EMR (69). In an analysis of 693 patients with granular mixed LSLs, rectal lesions measuring 4 cm or larger had a 22.1% risk of covert SMI and are therefore appropriate for ESD (71). Shahidi et al. described a rectum specific selective resection algorithm of patients who would benefit from en-bloc resection, which included lesions with a Kudo pit pattern Vi, non-granular LSLs Paris 0–Is or 0–IIa + Is, granular LSLs Paris 0–IIa + Is with a dominant nodule ≥10 mm (72).
In addition to the gross morphology, evaluation of the pit pattern by NBI magnifying endoscopy using the JNET pit pattern classification can help select patients who would benefit from en-bloc resection. In particular, JNET type 2B lesions, defined by an irregular vessel distribution and caliber and irregular surface pattern, correlate with high grade intramucosal neoplasia and shallow submucosal invasive cancer (73,74).
Despite the advances in endoscopic resection techniques, certain colorectal lesions are not amenable to resection via the standard endoscopic techniques. These include certain residual/recurrent fibrotic polyps or non-lifting lesions with extensive submucosal fibrosis, lesions involving difficult anatomical locations such as the appendiceal or diverticular orifice, post-polypectomy scars from incompletely resected malignant polyps, and indeterminate lesions for which diagnosis can inform management strategy. In addition, both EMR and ESD cannot effectively resect subepithelial lesions extending beyond the submucosa. EFTR enables transmural resection involving the mucosa to the muscularis propria allowing endoscopists to overcome these limitations. FTRD system can also be used to obtain full-thickness specimen of the intestinal wall in patients suspected of having a neuromuscular gut disorder such as Hirschsprung disease or enteric ganglionitis.
Direct comparisons between EMR/ESD and EFTR are difficult, as the majority of lesions resected by FTRD are deemed difficult or unresectable by other conventional techniques. A few studies reporting on ESD vs. EFTR of scar embedded polyps and recurrent colorectal neoplasia suggest comparable success rates (75,76) although procedural times are considerably longer for ESD, and EFTR may be favored in certain overlapping indications (77). However, historically reported R0 resection rates for EFTR have been lower than ESD likely due to the size limitation of the FTRD and severe fibrosis associated with lesions resected by EFTR. Ongoing research in a multicenter randomized controlled trial (CURE study) aims to determine if EFTR is superior in reducing recurrence.
Very large lesions (>3 cm) may not be appropriate for the FTRD system as the FTRD cap diameter of 21 mm and depth of 23 mm limits its efficacy for larger lesions. In the presence of scarring or malignant histology, lesions >2 cm should be avoided for en-bloc FTRD. The large cap size may also preclude reaching the target lesion especially in a narrow colonic lumen. However, various hybrid techniques have been proposed to overcome this limitation. In general, these techniques refer to using piecemeal EMR and ESD followed by FTRD of scarred or residual abnormal tissue. First, the lifting parts are resected with conventional EMR or ESD techniques. Afterwards, the residual non-lifting part is resected with the FTRD system. A recent single center experience included 75 patients who underwent hybrid EFTR (EMR/FTRD) for large (>25 mm) non-lifting colorectal adenomas with a mean lesion size of 36.5 mm. The technical success rate was 100% and macroscopic complete resection was 97.3%. Procedural adverse events were reported in 6.7% of cases leading to surgery in one patient with acute appendicitis (78). Hybrid EFTR appears to be a safe and effective approach extending the scope and application of EFTR in selected patients. Andrisani et al. also described the successful use of the FTRD system as a rescue procedure after difficult ESD (79).
Data on long-term recurrence after EFTR is limited as most studies are limited to 3 to 6 months. The German FTRD registry reports a recurrence rate of 13.5% after a median of 22 weeks and the WALL RESECT study reported a recurrence rate of 12.3% at 3 months after resection of difficult adenomas (80). The FTRD clip was reported to detach spontaneously around 70% of the cases at 3 months. Most recurrences can be successfully treated with re-EFTR, EMR, ESD, or avulsion (Figure 8).

Strengths and limitations of the review
This article provides an in-depth discussion of the technical aspects of EMR while highlighting more recent variations such as tip-in EMR and pre-cut EMR. It also provides an evidence-based review on the clinical applications of EFTR with a focus on the FTRD system. This review is limited by lack of data on direct comparisons between EMR, ESD and EFTR and between certain EMR variations. Therefore, in some cases, the ideal resection strategy is yet to be determined.
Conclusions
With the growth of the endoscopic armamentarium for colorectal lesions, surgical resection is currently rarely indicated apart from deep submucosal carcinoma. EMR remains the cornerstone for management of large colorectal lesions and many of the traditional limitations of EMR can now be overcome with the expanding endoscopic toolkit and improved EMR outcomes.
The role of FTRD has expanded in recent years and has become a crucial addition to the endoscopic tools for resection of colorectal lesion reducing the need for more invasive surgery. The combination of FTRD with other resection techniques such as EMR and ESD have further expanded the indications. Determining the optimal resection strategy for certain colorectal lesions remains challenging and future studies may help further define the role of each technique within the growing endoscopic toolkit.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Terry L. Jue) for the series “A U. S. Perspective on Endoscopic Resection of Neoplastic Lesions of the Gastrointestinal Tract” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-24-10/rc
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-24-10/prf
Funding: None.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-24-10/coif). The series “A U. S. Perspective on Endoscopic Resection of Neoplastic Lesions of the Gastrointestinal Tract” was commissioned by the editorial office without any funding or sponsorship. P.S.G. is a consultant for Boston Scientific, Fujifilm Medical Systems, Olympus America, Alira Health, Neptune Medical and Ovesco Endoscopy USA. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and in ensuring that questions related to the accuracy or integrity of any part of the work presented have been appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Baxter NN, Warren JL, Barrett MJ, et al. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol 2012;30:2664-9. [Crossref] [PubMed]
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977-81. [Crossref] [PubMed]
- Ngamruengphong S, Pohl H, Haito-Chavez Y, et al. Update on Difficult Polypectomy Techniques. Curr Gastroenterol Rep 2016;18:3. [Crossref] [PubMed]
- Soetikno RM, Gotoda T, Nakanishi Y, et al. Endoscopic mucosal resection. Gastrointest Endosc 2003;57:567-79. [Crossref] [PubMed]
- Tanaka S, Oka S, Kaneko I, et al. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc 2007;66:100-7. [Crossref] [PubMed]
- Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008;68:1066-72. [Crossref] [PubMed]
- ROSENBERG N. Submucosal saline wheal as safety factor in fulguration or rectal and sigmoidal polypi. AMA Arch Surg 1955;70:120-2. [Crossref] [PubMed]
- Deyhle P, Jenny S, Fumagalli I. Endoscopic polypectomy in the proximal colon. A diagnostic, therapeutic (and preventive?) intervention. Dtsch Med Wochenschr 1973;98:219-20. [Crossref] [PubMed]
- Soetikno R, Kaltenbach T. Dynamic submucosal injection technique. Gastrointest Endosc Clin N Am 2010;20:497-502. [Crossref] [PubMed]
- Yamamoto H, Yahagi N, Oyama T, et al. Usefulness and safety of 0.4% sodium hyaluronate solution as a submucosal fluid "cushion" in endoscopic resection for gastric neoplasms: a prospective multicenter trial. Gastrointest Endosc 2008;67:830-9. [Crossref] [PubMed]
- Arantes V, Albuquerque W, Benfica E, et al. Submucosal injection of 0.4% hydroxypropyl methylcellulose facilitates endoscopic mucosal resection of early gastrointestinal tumors. J Clin Gastroenterol 2010;44:615-9. [Crossref] [PubMed]
- Arezzo A, Pagano N, Romeo F, et al. Hydroxy-propyl-methyl-cellulose is a safe and effective lifting agent for endoscopic mucosal resection of large colorectal polyps. Surg Endosc 2009;23:1065-9. [Crossref] [PubMed]
- Moss A, Bourke MJ, Metz AJ. A randomized, double-blind trial of succinylated gelatin submucosal injection for endoscopic resection of large sessile polyps of the colon. Am J Gastroenterol 2010;105:2375-82. [Crossref] [PubMed]
- Ferreira AO, Moleiro J, Torres J, et al. Solutions for submucosal injection in endoscopic resection: a systematic review and meta-analysis. Endosc Int Open 2016;4:E1-E16. [PubMed]
- Rex DK, Lahr RE. The demise of ORISE, a submucosal lifting agent for endoscopic resection. AME Case Rep 2023;7:11. [Crossref] [PubMed]
- Bar-Yishay I, Shahidi N, Gupta S, et al. Outcomes of Deep Mural Injury After Endoscopic Resection: An International Cohort of 3717 Large Non-Pedunculated Colorectal Polyps. Clin Gastroenterol Hepatol 2022;20:e139-47. [Crossref] [PubMed]
- Holt BA, Jayasekeran V, Sonson R, et al. Topical submucosal chromoendoscopy defines the level of resection in colonic EMR and may improve procedural safety (with video). Gastrointest Endosc 2013;77:949-53. [Crossref] [PubMed]
- Hirao M, Hatakeyama H, Asanuma T, et al. Endoscopic resection with local injection of hypertonic saline epinephrine for the treatment of early gastric cancer. Gan To Kagaku Ryoho 1988;15:1466-72. [PubMed]
- Tullavardhana T, Akranurakkul P, Ungkitphaiboon W, et al. Efficacy of submucosal epinephrine injection for the prevention of postpolypectomy bleeding: A meta-analysis of randomized controlled studies. Ann Med Surg (Lond) 2017;19:65-73. [Crossref] [PubMed]
- Li D, Wang W, Xie J, et al. Efficacy and safety of three different endoscopic methods in treatment of 6-20 mm colorectal polyps. Scand J Gastroenterol 2020;55:362-70. [Crossref] [PubMed]
- van Hattem WA, Shahidi N, Vosko S, et al. Piecemeal cold snare polypectomy versus conventional endoscopic mucosal resection for large sessile serrated lesions: a retrospective comparison across two successive periods. Gut 2021;70:1691-7. [Crossref] [PubMed]
- McWhinney CD, Vemulapalli KC, El Rahyel A, et al. Adverse events and residual lesion rate after cold endoscopic mucosal resection of serrated lesions ≥10 mm. Gastrointest Endosc 2021;93:654-9. [Crossref] [PubMed]
- Thoguluva Chandrasekar V, Aziz M, Patel HK, et al. Efficacy and Safety of Endoscopic Resection of Sessile Serrated Polyps 10 mm or Larger: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2020;18:2448-2455.e3. [Crossref] [PubMed]
- Rashid M, Swartz A, Alomari M, et al. Cold vs hot EMR for large colon polyps (≥20 mm); analysis of effectiveness, safety and cost-benefit. Gastrointestinal Endoscopy 2023;97:AB535-6. [Crossref]
- Kim HG, Thosani N, Banerjee S, et al. Underwater endoscopic mucosal resection for recurrences after previous piecemeal resection of colorectal polyps (with video). Gastrointest Endosc 2014;80:1094-102. [Crossref] [PubMed]
- Friedland S, Shelton A, Kothari S, et al. Endoscopic management of nonlifting colon polyps. Diagn Ther Endosc 2013;2013:412936. [Crossref] [PubMed]
- Yamashina T, Hanaoka N, Setoyama T, et al. Efficacy of Underwater Endoscopic Mucosal Resection for Nonpedunculated Colorectal Polyps: A Systematic Review and Meta-Analysis. Cureus 2021;13:e17261. [Crossref] [PubMed]
- Yoshida N, Inoue K, Dohi O, et al. Precutting EMR with full or partial circumferential incision with a snare tip for the en bloc resection of difficult colorectal lesions. VideoGIE 2018;3:378-80. [Crossref] [PubMed]
- Yoshii S, Kikuchi T, Hayashi Y, et al. Hybrid Endoscopic Resection With Endo-knife and Snare for Colorectal Lesions: A Systematic Review and Meta-analysis. Techniques and Innovations in Gastrointestinal Endoscopy 2023;25:135-45. [Crossref]
- Oh CK, Cho YS, Lee SH, et al. Anchoring endoscopic mucosal resection versus conventional endoscopic mucosal resection for large nonpedunculated colorectal polyps: a randomized controlled trial. Endoscopy 2023;55:158-64. [Crossref] [PubMed]
- Burgess NG, Metz AJ, Williams SJ, et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol 2014;12:651-61.e1-3.
- Fahrtash-Bahin F, Holt BA, Jayasekeran V, et al. Snare tip soft coagulation achieves effective and safe endoscopic hemostasis during wide-field endoscopic resection of large colonic lesions (with videos). Gastrointest Endosc 2013;78:158-163.e1. [Crossref] [PubMed]
- Conio M, Repici A, Demarquay JF, et al. EMR of large sessile colorectal polyps. Gastrointest Endosc 2004;60:234-41. [Crossref] [PubMed]
- Luigiano C, Consolo P, Scaffidi MG, et al. Endoscopic mucosal resection for large and giant sessile and flat colorectal polyps: a single-center experience with long-term follow-up. Endoscopy 2009;41:829-35. [Crossref] [PubMed]
- Rieder E, Martinec DV, Dunst CM, et al. A novel technique for natural orifice endoscopic full-thickness colon wall resection: an experimental pilot study. J Am Coll Surg 2011;213:422-9. [Crossref] [PubMed]
- Schurr MO, Baur FE, Krautwald M, et al. Endoscopic full-thickness resection and clip defect closure in the colon with the new FTRD system: experimental study. Surg Endosc 2015;29:2434-41. [Crossref] [PubMed]
- Valli PV, Kaufmann M, Vrugt B, et al. Endoscopic resection of a diverticulum-arisen colonic adenoma using a full-thickness resection device. Gastroenterology 2014;147:969-71. [Crossref] [PubMed]
- Schmidt A, Damm M, Caca K. Endoscopic full-thickness resection using a novel over-the-scope device. Gastroenterology 2014;147:740-742.e2. [Crossref] [PubMed]
- Schmidt A, Bauerfeind P, Gubler C, et al. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy 2015;47:719-25. [Crossref] [PubMed]
- Schmidt A, Beyna T, Schumacher B, et al. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut 2018;67:1280-9. [Crossref] [PubMed]
- Zwager LW, Bastiaansen BAJ, Bronzwaer MES, et al. Endoscopic full-thickness resection (eFTR) of colorectal lesions: results from the Dutch colorectal eFTR registry. Endoscopy 2020;52:1014-23. [Crossref] [PubMed]
- Kuellmer A, Mueller J, Caca K, et al. Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc 2019;89:1180-1189.e1. [Crossref] [PubMed]
- Zwager LW, Bastiaansen BAJ, van der Spek BW, et al. Endoscopic full-thickness resection of T1 colorectal cancers: a retrospective analysis from a multicenter Dutch eFTR registry. Endoscopy 2022;54:475-85. [Crossref] [PubMed]
- Coronel E, Tillman M, Nehme F, et al. Outcomes of endoscopic full thickness resection for formal tumor staging among incompletely resected malignant colorectal lesions: a US academic cancer center experience. Gastrointestinal Endoscopy 2023;97:AB774-5. [Crossref]
- Ichkhanian Y, Barawi M, Seoud T, et al. Endoscopic full-thickness resection of polyps involving the appendiceal orifice: a multicenter international experience. Endoscopy 2022;54:16-24. [Crossref] [PubMed]
- Meier B, Albrecht H, Wiedbrauck T, et al. Full-thickness resection of neuroendocrine tumors in the rectum. Endoscopy 2020;52:68-72. [Crossref] [PubMed]
- Meier B, Stritzke B, Kuellmer A, et al. Efficacy and Safety of Endoscopic Full-Thickness Resection in the Colorectum: Results From the German Colonic FTRD Registry. Am J Gastroenterol 2020;115:1998-2006. [Crossref] [PubMed]
- Dolan RD, Bazarbashi AN, McCarty TR, et al. Endoscopic full-thickness resection of colorectal lesions: a systematic review and meta-analysis. Gastrointest Endosc 2022;95:216-224.e18. [Crossref] [PubMed]
- Repici A, Pellicano R, Strangio G, et al. Endoscopic mucosal resection for early colorectal neoplasia: pathologic basis, procedures, and outcomes. Dis Colon Rectum 2009;52:1502-15. [Crossref] [PubMed]
- Tanaka S, Kashida H, Saito Y, et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2020;32:219-39. [Crossref] [PubMed]
- Tateishi Y, Nakanishi Y, Taniguchi H, et al. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol 2010;23:1068-72. [Crossref] [PubMed]
- Yamaoka Y, Shiomi A, Kagawa H, et al. Lymph node metastasis in T1 colorectal cancer with the only high-risk histology of submucosal invasion depth ≥ 1000 µm. Int J Colorectal Dis 2022;37:2387-95. [Crossref] [PubMed]
- Kitajima K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 2004;39:534-43. [Crossref] [PubMed]
- Takada K, Hotta K, Imai K, et al. Tip-in EMR as an alternative to endoscopic submucosal dissection for 20- to 30-mm nonpedunculated colorectal neoplasms. Gastrointest Endosc 2022;96:849-856.e3. [Crossref] [PubMed]
- Yamashina T, Uedo N, Akasaka T, et al. Comparison of Underwater vs Conventional Endoscopic Mucosal Resection of Intermediate-Size Colorectal Polyps. Gastroenterology 2019;157:451-461.e2. [Crossref] [PubMed]
- Tate DJ, Bahin FF, Desomer L, et al. Cold-forceps avulsion with adjuvant snare-tip soft coagulation (CAST) is an effective and safe strategy for the management of non-lifting large laterally spreading colonic lesions. Endoscopy 2018;50:52-62. [PubMed]
- Kumar V, Broadley H, Rex DK. Safety and efficacy of hot avulsion as an adjunct to EMR (with videos). Gastrointest Endosc 2019;89:999-1004. [Crossref] [PubMed]
- Belderbos TD, Leenders M, Moons LM, et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014;46:388-402. [Crossref] [PubMed]
- Pellise M, Burgess NG, Tutticci N, et al. Endoscopic mucosal resection for large serrated lesions in comparison with adenomas: a prospective multicentre study of 2000 lesions. Gut 2017;66:644-53. [Crossref] [PubMed]
- Rex KD, Vemulapalli KC, Rex DK. Recurrence rates after EMR of large sessile serrated polyps. Gastrointest Endosc 2015;82:538-41. [Crossref] [PubMed]
- Moss A, Williams SJ, Hourigan LF, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut 2015;64:57-65. [Crossref] [PubMed]
- Tate DJ, Desomer L, Argenziano ME, et al. Treatment of adenoma recurrence after endoscopic mucosal resection. Gut 2023;72:1875-86. [Crossref] [PubMed]
- Gupta S, Lieberman D, Anderson JC, et al. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020;158:1131-1153.e5. [Crossref] [PubMed]
- Kemper G, Turan AS, Schoon EJ, et al. Endoscopic techniques to reduce recurrence rates after colorectal EMR: systematic review and meta-analysis. Surg Endosc 2021;35:5422-9. [Crossref] [PubMed]
- Rex DK, Haber GB, Khashab M, et al. Snare Tip Soft Coagulation vs Argon Plasma Coagulation vs No Margin Treatment After Large Nonpedunculated Colorectal Polyp Resection: a Randomized Trial. Clin Gastroenterol Hepatol 2024;22:552-561.e4. [Crossref] [PubMed]
- Tanaka S, Saitoh Y, Matsuda T, et al. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2021;56:323-35. [Crossref] [PubMed]
- Ohata K, Kobayashi N, Sakai E, et al. Long-term Outcomes After Endoscopic Submucosal Dissection for Large Colorectal Epithelial Neoplasms: A Prospective, Multicenter, Cohort Trial From Japan. Gastroenterology 2022;163:1423-1434.e2. [Crossref] [PubMed]
- Yamada M, Saito Y, Sakamoto T, et al. Endoscopic predictors of deep submucosal invasion in colorectal laterally spreading tumors. Endoscopy 2016;48:456-64. [Crossref] [PubMed]
- Bogie RMM, Veldman MHJ, Snijders LARS, et al. Endoscopic subtypes of colorectal laterally spreading tumors (LSTs) and the risk of submucosal invasion: a meta-analysis. Endoscopy 2018;50:263-82. [Crossref] [PubMed]
- D'Amico F, Amato A, Iannone A, et al. Risk of Covert Submucosal Cancer in Patients With Granular Mixed Laterally Spreading Tumors. Clin Gastroenterol Hepatol 2021;19:1395-401. [Crossref] [PubMed]
- Shahidi N, Vosko S, Gupta S, et al. A Rectum-Specific Selective Resection Algorithm Optimizes Oncologic Outcomes for Large Nonpedunculated Rectal Polyps. Clin Gastroenterol Hepatol 2023;21:72-80.e2. [Crossref] [PubMed]
- Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc 2016;28:526-33. [Crossref] [PubMed]
- Kobayashi S, Yamada M, Takamaru H, et al. Diagnostic yield of the Japan NBI Expert Team (JNET) classification for endoscopic diagnosis of superficial colorectal neoplasms in a large-scale clinical practice database. United European Gastroenterol J 2019;7:914-23. [Crossref] [PubMed]
- Azzolini F, Camellini L, Sassatelli R, et al. Endoscopic submucosal dissection of scar-embedded rectal polyps: a prospective study (Esd in scar-embedded rectal polyps). Clin Res Hepatol Gastroenterol 2011;35:572-9. [Crossref] [PubMed]
- Hurlstone DP, Shorthouse AJ, Brown SR, et al. Salvage endoscopic submucosal dissection for residual or local recurrent intraepithelial neoplasia in the colorectum: a prospective analysis. Colorectal Dis 2008;10:891-7. [Crossref] [PubMed]
- Valli PV, Pohl D, Fried M, et al. Diagnostic use of endoscopic full-thickness wall resection (eFTR)-a novel minimally invasive technique for colonic tissue sampling in patients with severe gastrointestinal motility disorders. Neurogastroenterol Motil 2018; [Crossref] [PubMed]
- Meier B, Elsayed I, Seitz N, et al. Efficacy and safety of combined EMR and endoscopic full-thickness resection (hybrid EFTR) for large nonlifting colorectal adenomas. Gastrointest Endosc 2023;98:405-11. [Crossref] [PubMed]
- Andrisani G, Di Matteo FM. Hybrid resection with ESD and FTRD: Could this be a rescue treatment in the presence of severe submucosal fibrosis? Dig Liver Dis 2019;51:607-9. [Crossref] [PubMed]
- Krutzenbichler I, Dollhopf M, Diepolder H, et al. Technical success, resection status, and procedural complication rate of colonoscopic full-wall resection: a pooled analysis from 7 hospitals of different care levels. Surg Endosc 2021;35:3339-53. [Crossref] [PubMed]
Cite this article as: Nehme F, Ge PS. Endoscopic mucosal resection and full-thickness resection of neoplastic lesions of the colon: clinical application and techniques—a narrative review. Ann Laparosc Endosc Surg 2025;10:15.


