Laparoscopic spleen-preserving complete splenic hilum lymphadenectomy for advanced proximal gastric cancer
Introduction
With the improvement of minimally invasive technique and its potential minimally invasive advantages, laparoscopic radical distal gastrectomy is more and more popular, especially in East Asia. The short-term outcomes of CLASS-01 trial were encouraging and demonstrated the safety and feasibility of laparoscopic distal gastrectomy for advanced gastric cancer (1). Total gastrectomy with standard D2 lymph nodes (LNs) dissection including LNs along splenic artery and splenic hilum dissection is recommended in the 3rd version of Japanese gastric cancer treatment guidelines for advanced middle and upper gastric cancer (2). Nevertheless, because of the deep location of spleen, circuitous splenic vessels and easy splenic and pancreatic parenchyma damage, complete removal of the LNs along splenic artery and splenic hilum is technically demanding. In last century, splenectomy with partial pancreatectomy was recommended to remove these regional LNs, but it resulted in terrible complication and death rate and there were no survival benefits. As a consequence, the clinical significance of this procedure was controversial.
Spleen-preserving splenic hilum lymphadenectomy has been gradually accepted because of the advancement of anatomical comprehension and the improvement of surgical techniques (3). Nevertheless, laparoscopic total gastrectomy with D2 lymphadenectomy was yet not widely performed due to the technical difficulty of spleen- and pancreas-preserving splenic hilum lymphadenectomy and totally laparoscopic Roux-en-Y esophagojejunostomy. Herein, we present our technical characteristics and initial experience of laparoscopic radical total gastrectomy with spleen-preserving complete splenic hilum lymphadenectomy for advanced gastric cancer.
Patient selection
The inclusion criteria of present study were described as follows: primary gastric adenocarcinoma confirmed pathologically by endoscopic biopsy; tumor was located in the upper and middle third of stomach; preoperative TNM stage was T1–4aN0–3M0 according to 7th Edition AJCC Cancer Staging Manual based on computer tomography and endoscopic ultrasonography. Patients proved with distant metastases or obvious invading of adjacent organ based on preoperative computer tomography or intraoperative exploration were not suitable for this procedure. An informed consent about the potentially surgical risks and the technical details of operation was provided for every patient. The protocol of this procedure obtained the approval from Guangdong Provincial Hospital of Chinese Medicine Ethics Committee.
Pre-operative preparation
Pre-operative preparation was performed according to the concept of fast track surgery. No normal bowel preparation was used and patients were fasting one night before operation. Intravenous antibiotics were infused 30 minutes before operation.
Equipment preference
Operative equipment contain: HD camera and display system, pneumoperitoneum machine, laparoscopic standard instruments, ultrasonic scalpel, circular stapler, linear stapler and other conventional surgical instruments.
Surgical technique
The local LNs in this procedure were defined as the 3rd edition of Japanese Classification of Gastric Carcinoma (4).
After general anesthesia, patient was placed in a supine position with legs apart and 20° head-up tilt. A standard method of five trocars was used (5). The dominant surgeon stood at the left side of patient with the assistant at the right side and the camera holder between the legs. The CO2 pneumoperitoneum was established and maintained at a pressure of 12 to 15 mmHg. The procedure was performed as our previous study (5). While performing No. 10 and 11 LNs dissection, these special LNs were divided into two parts, namely anterosuperior LNs and posterior LNs according to the position relationship with splenic vessels (6). Before operation, the intraperitoneal exploration was performed to examine the primary tumor and know if there were distant metastases.
The gastrocolic ligament was dissected firstly at the left part and divided from left to right until to the inferior region of pylorus. Then the pedicle of right gastroepiploic artery and vein were exposed and ligated with the infrapyloric fatty tissue removed. And the No. 4d and No. 6 LNs were dissected.
The plica gastropancreatica was exposed through over-turning the body of the stomach cranially and ventrally by the assistant surgeon. With the introduction of right gastroepiploic artery, the gastroduodenal artery was indentified. Along the pathway of the gastroduodenal artery, the supra-pancreas area was exposed and the proper hepatic artery and common hepatic artery were traced with the left gastric vessel and splenic artery indentified. These primary vessels were ligated at roots and the fatty tissue along them was removed as well as the tissue along the celiac trunk. As a consequence, No. 7, 8a, 9 and 12a LNs were completely removed. Then, the duodenohepatic ligament and gastrohepatic ligament were dissected and the No. 1, 3 and 5 LNs along the lesser gastric curvature were removed.
Anterosuperior No. 10 and 11 LNs dissection
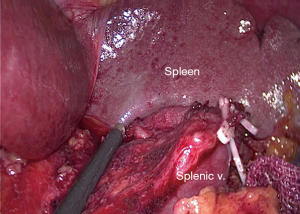
The location of the surgeons was adjusted. The dominant surgeon stood between legs and the camera holder stood at beside the patient’s right leg. The camera view was focused on the inferior pole of spleen. The greater curvature of the stomach was overturned cranially and ventrally to make the gastrosplenic ligament under an appropriate tension. The left gastroepiploic vessels and short gastroepiploic vessels were exposed and ligated at root with No. 4sb and 4sa LNs dissection. Along the left gastroepiploic vessels, the splenic vessels and the superior border of pancreas were exposed. The sheath of the splenic artery trunk was dissected. Then the tissue anterosuperior to the splenic vessel and splenic hilum was removed along the pancreatic tail from the vessel trunk to its terminal branches with the posterior gastric vessels ligation. The anterosuperior No. 11 and 10 LNs were completely dissected (Figure 1).
After anterosuperior No. 10 and 11 LNs dissection, the linear staplers were used to transect the duodenum 2 cm distal to the pylorus and the esophagus with adequate tumor free margin. Then the specimen extraction was performed with a midline mini-incision.
Posterior No. 10 and 11 LNs dissection
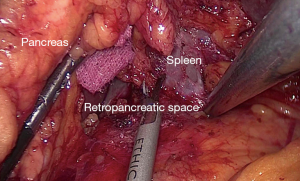
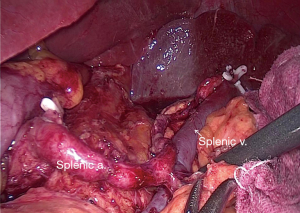
After specimen extraction, the pneumoperitoneum was rebuilt and the dominant surgeon exchanged to the right side. The distal pancreas was completely mobilized by separating the fascia between the retroperitoneum and the pancreatic tail (Figure 2) (5). After exposing the retro-pancreatic space, the posterior paries of splenic vein was exactly identified. The soft tissue between splenic vein and artery and the LNs posterior to the splenic vessels was completely removed by skeletonizing the splenic vessels with the great pancreatic artery and the pancreatic tail artery preserved. At last, posterior No. 10 and 11 LNs were dissected with all splenic vessel branches saved (Figure 3).
After laparoscopic radical total gastrectomy with spleen-preserving complete splenic hilum lymphadenectomy, totally laparoscopic Roux-en-Y esophagojejunostomy was performed with circular stapler and the jejunojejunostomy was carried out in a side-to-side fashion using linear stapler.
Role of team members
Our multi-disciplinary team consisted of surgeons, iconography doctors, anesthetists, physicians of oncology, nurses, pathologists and other allied health professionals. The pre-operative examination and evaluation were performed by surgeons and iconography doctors. The specimen evaluation was performed by surgeons and pathologists. Peri-operative nursing care and management was performed by nurses and surgeons. Postoperative adjuvant chemotherapy was performed by physicians of oncology if necessary.
Post-operative management
Post-operative management was performed according to the concept of fast track surgery. One more time of intravenous antibiotics was performed within 24 hours after operation. The gastric tube and urinary catheter were removed on the first post-operative day and liquid diet was beginning at the same time. Ambulation as early as possible was advocated.
Tips, tricks and pitfalls
Laparoscopic total gastrectomy for advanced gastric cancer still confronted with many controversies, including morbidity, adequate tumor margin, standard lymphadenectomy and long-term survival, especially complete peri-splenic hilum (containing No. 10 and 11d) LNs dissection for advanced proximal tumor. Due to the technical difficulties in complete removal of peri-splenic hilum LNs, only a fewer surgeons in East Asian, such as China, Korea and Japan, focused on laparoscopic radical total gastrectomy with spleen-preserving complete splenic hilum lymphadenectomy.
As the characteristics of laparoscopic technique, laparoscopic splenic hilum lymphadenectomy has its technical difficulty, but also has its special advantages. In laparoscopic gastrectomy, only four work ports were used, so the traction is lacking and the exposure is poor. It is easy to affect the operation and increases the unexpected injury rate to splenic vessel, parenchyma of the pancreas and spleen. However, minimally invasive surgery has a valid magnifying effection. When performing laparoscopic lymphadenectomy along peri-splenic area, surgeons can clearly identify all various branches of the splenic vessels, as well terminal branches, so that they can effectively reduce the accident of vascular injury as well as reducing the rate of damage to the pancreas and spleen.
In our initial experience, the postoperative outcomes showed that the mean operative time was slightly longer than other relative studies (7-9). One of the reasons may be that the small sample of our series study and we have not crossed over the learning curve and another one reason may be our more complete removal of the splenic hilum LNs compared with other studies. However, the other short-term outcomes containing blood loss, number of LNs harvested and rate of postoperative complication were satisfactory. Therefore, laparoscopic spleen-preserving complete peri-splenic hilum lymphadenectomy was safe and technically feasible.
When No. 10 and 11 LNs dissected, the anterosuperior and posterior portions of No. 10 and 11 LNs were dissected respectively. After the left gastroepiploic vessels and short gastroepiploic vessels were ligated at roots, the splenic artery and vein were exposed and identified. No. 10 and No. 11 anterosuperior LNs were dissected from the splenic vessel primary trunk to its branches with the introduction of the splenic vessel.
Compared with the anterosuperior LNs dissection, the posterior LNs dissection was more difficult and technically demanding. The retropancreatic space was deep and narrow and the pancreas could not be easily grasped make it difficult to achieve completely removal of these special LNs. Our strategy was separating the retropancreatic space with priority and using the suction/irrigation tube for traction. And the posterior parietes of splenic vessel were completely exposed so that we could easily remove the posterior LNs. Opening the sheath of splenic artery and skeletonizing the spleni vessel from the primary trunk to its branches, the soft tissue along splenic vessels could be dissected by entering vascular intrathecal space. During operation, surgeons must keep visual field clear with the irrigation and suction technique and the small bleeding trauma could be handled with electrocoagulation and attraction.
In laparoscopic splenic hilum lymphadenectomy, skilled master of the ultrasonic scalpel was very important. When dissection, the non-functional face should be near to the key structures, which is an effective way to avoid damaging the pancreas, spleen and splenic vessels. Because of the tortuous splenic vessel, exposure of the splenic hilum area was difficult. We used an elastic band for traction which made lymphadenectomy safer and easier. In our series, one case of intro-operative splenic vessel injury was occurred and handled with laparoscopic vessel suture technique successfully. When unexpected bleeding occurred, surgeons must stay calm and keep the operative field clear using aspirator and skillful laparoscopic suture and knot tying technique were necessary. In addition, operative team building is important for this difficult laparoscopic operation.
In this laparoscopic procedure, surgeons must well understand the complex variations of splenic vessels trunk and its branches and different pancreatic parenchyma and shape. These variations might increase the injury rate of splenic vessels and the possibility of unexpected pancreatic fistula. The distance from the tail of pancreas to spleen and the difference of splenic lobar vessels must be carefully identified when performing splenic hilum LNs dissection. Generally, splenic artery was divided into several branches at the lienorenal ligament. Some scholars explored the different anatomical variations of the splenic artery and its clinical implications in 320 cadavers (10). They clearly suggested that there was variation in terminal distribution pattern of the splenic artery. In 9 (2.8%) cadavers, the splenic artery passed through the splenic hilum without diving. The splenic artery divided into terminal branches in 311 (97%) cadavers, containing two terminal branches (63.1%), the most common fashion followed by four branches (18.8%), six branches (9.7%) and more than six branches (5.6%). Apparently, the more terminal branches the splenic artery divided into, the more demanding of splenic hilum lymphadenectomy might be. To identify the fat tissue or pancreatic tissue carefully was also very significant.
There were portion of No. 10 and No. 11 LNs deeply behind the pancreas. By separating retro-pancreatic space, complete removal of these LNs was simple and easy. In our series, the posterior portion of No. 10 and 11 LNs were dissected and examined alone. Several previous reports have demonstrated that the metastasis rate of No. 10 LNs was 9–20.9% in proximal gastric cancer (11-14). However, there were still no study about the metastasis rate of posterior portion of No. 10 and 11 LNs. Therefore, future studies about the metastasis rate and its clinical significance of these special LNs dissection are needed (6).
In conclusion, for skilled laparoscopic surgeon, laparoscopic total gastrectomy and D2 lymphadenectomy with spleen-preserving complete splenic hilum LNs dissection is safe and technically feasible.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.11.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu Y, Huang C, Sun Y, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol 2016;34:1350-7. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Wang W, Liu Z, Xiong W, et al. Totally laparoscopic spleen-preserving splenic hilum lymph nodes dissection in radical total gastrectomy: an omnibearing method. Surg Endosc 2016;30:2030-5. [Crossref] [PubMed]
- Wang W, Xiong W, Liu Z, et al. Clinical significance of No. 10 and 11 lymph nodes posterior to the splenic vessel in D2 radical total gastrectomy: An observational study. Medicine (Baltimore) 2016;95:e4581 [Crossref] [PubMed]
- Mou TY, Hu YF, Yu J, et al. Laparoscopic splenic hilum lymph node dissection for advanced proximal gastric cancer: a modified approach for pancreas- and spleen-preserving total gastrectomy. World J Gastroenterol 2013;19:4992-9. [Crossref] [PubMed]
- Hyung WJ, Lim JS, Song J, et al. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg 2008;207:e6-11. [Crossref] [PubMed]
- Jia-Bin W, Chang-Ming H, Chao-Hui Z, et al. Laparoscopic spleen-preserving No. 10 lymph node dissection for advanced proximal gastric cancer in left approach: a new operation procedure. World J Surg Oncol 2012;10:241. [Crossref] [PubMed]
- Pandey SK, Bhattacharya S, Mishra RN, et al. Anatomical variations of the splenic artery and its clinical implications. Clin Anat 2004;17:497-502. [Crossref] [PubMed]
- Sasada S, Ninomiya M, Nishizaki M, et al. Frequency of lymph node metastasis to the splenic hilus and effect of splenectomy in proximal gastric cancer. Anticancer Res 2009;29:3347-51. [PubMed]
- Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg 2006;93:559-63. [Crossref] [PubMed]
- Mönig SP, Collet PH, Baldus SE, et al. Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol 2001;76:89-92. [Crossref] [PubMed]
- Maruyama K, Gunvén P, Okabayashi K, et al. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg 1989;210:596-602. [Crossref] [PubMed]
Cite this article as: Wang W, Xiong W, Peng Q, Ye S, Zheng Y, Luo L, Tan P, Wan J. Laparoscopic spleen-preserving complete splenic hilum lymphadenectomy for advanced proximal gastric cancer. Ann Laparosc Endosc Surg 2016;1:23.