
Laparoscopic right hemicolectomy with transvaginal specimen extraction
Introduction
Laparoscopic surgery is a well-established choice for the patients diagnosed with colon diseases. However, this technique inevitably needs an abdominal incision to extract the specimen and complete anastomosis, which could cause some incision-associated complications, such as infection and hernia. Besides, such an incision weakens the cosmetic effects to some extent. In order to minimize the trauma associated with the abdominal incision, natural orifice transluminal surgery (NOTES) has gain popularity among surgeons. This technique could avoid the bodily injury and decrease the incision-associated complications (1,2). Nevertheless, in the present, lack of reliable platforms and instruments and reliable methods to close the natural orifice incision, the complex procedures, high-selective operative approaches, and unclear exposure of surgical field, are obstacles to applying the NOTES (3,4).
Combining the notion of laparoscopic techniques with that of specimen extraction via natural orifice can sustain the advantages of both laparoscopic surgery and NOTES. It may bridge the gap between laparoscopy and NOTES. The vagina is regarded as a suitable route for natural orifice surgery and specimen extraction, considering the improved healing and elasticity compared to anus (5-10). Moreover, very rare studies related to laparoscopic right hemicolectomy with transvaginal specimen extraction are available. Here, we have introduced this new procedure of “laparoscopic right hemicolectomy with transvaginal specimen extraction” (11).
Patient selection and work-up
- The lesions are located at ascending colon and sex is female;
- The depth of tumor invasion is clinically less than T4;
- The maximal diameter of the lesion is less than 8 cm;
- Body mass index (BMI) is less than 30 kg/m2.
Pre-operative preparation
All the patients took oral laxatives in the evening the day before the surgery, and an enema was given three times in the morning on the day of surgery. During anesthetic induction, a single dose of second-generation cephalosporin’s or levofloxacin was given by intravenous infusion. Such administration would be repeated if the operative time was more than three hours. All patients were given general anesthesia.
Equipment preference card
Laparoscope, regular general surgery equipment, ultrasound knife, 60 mm linear stapler, vaginal suture thread, sterile protection sleeve and uterine manipulator.
Procedure
The patient was placed in the split and left-title position with a pneumoperitoneum of 12 mmHg after the general anesthesia. The patients’ arms were tucked at the side and their shoulders were securely taped to the operating table. The operator stood on the left of the patient, the first assistant stood on the right of the patient, and the second assistant stood between the legs of the patient. The laparoscopic camera was inserted through a 10 mm trocar near the belly button. The 12 mm trocar was placed slightly above the 5 cm left of umbilicus. Three 5-mm trocars were placed at the position of lower right quadrant, the insertion points of the umbilical line with the left and right midclavicular line, respectively. No metastasis, dissemination and multiple primary tumor was found in patients with cancer and the lesions were located at the ascending colon. We operated in the plane between the duodenum, head of pancreas and the mesocolon. Start to cut up the ascending mesocolon along superior mesenteric artery to the origin of ileocolic artery and vein. Dissection is continued through the Toldt space up to the lower edge of the pancreas, where the right colic artery and vein are observed and ligated. Then, the great omentum was divided carefully and the right branch of the middle colic artery and vein was ligated and dissected. Dissect the fatty tissue clearly on the wall of ileum at the planned anastomosis location. The great omentum was divided as a further step. The lymph nodes around the vessels were removed completely. Dissect the epiploic appendages clearly on the wall of transverse colon at the planned anastomosis location. The endo GIA linear stapler inserted through the 12 mm trocar transected the transverse colon. Division was continued along the Toldt space and the lateral attachments of the ascending colon with the abdominal wall were released. The endo GIA linear stapler inserted transected the terminal of the ileum. A small incision was made at the stump of the colon and ileum, respectively. Another endo GIA linear stapler was used to perform the functional end-to-end anastomosis and then enclose the stump of the transverse colon and ileum. After sterilizing the vagina with diluted povidone-iodine solution, the most visible bulging part of the posterior vagina was cut under laparoscopic guidance, the protective sleeve was inserted, and then the specimen was extracted via vagina. Warm distilled water flushed abdominal cavity. One drainage tube was placed at the side of anastomosis and the other drainage tube was placed at hepatorenal recess through the trocars in the right quadrant. The pneumoperitoneum was released and the holes of trocar were sutured. Till then, the operation had been finished. The picture of a patient’s abdomen was presented (Figures 1,2).
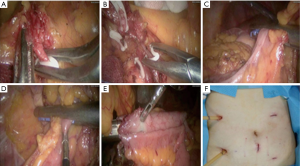
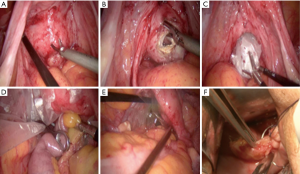
Role of team members
It is of great importance that an experienced team consisting of a colorectal surgeon, orthopedic oncologic surgeon, spine surgeon, urologist, plastic surgeon, vascular surgeon, musculoskeletal radiologist, medical oncologist, radiation oncologist, and specialized anesthesiologist evaluate and surgically treat tumors that are large and extend to or destroy the hemipelvis or the upper half of the sacrum.
Post-operative management
Adequate pain control is necessary to maximize cardiac and respiratory function and decrease the risk of complications. Without large incision, patients feel less pain than traditional laparoscopic surgery.
As a result of surgical stress, there is an increase in renin, aldosterone, and antidiuretic hormone release and activation of the sympathetic system resulting in sequestration of fluid (third spacing) and increased volume requirements.
The resumption of a diet is critical to the recovery of the patient undergoing intestinal surgery. Before discharge, it is accepted that patients should tolerate oral analgesia, not require IV hydration, and demonstrate return of intestinal tract function.
Tips, tricks and pitfalls
Anatomy of superior mesenteric vein and Helen trunk and anterior pancreas-duodenum fascia is of vital important for right hemicolectomy. Laparoscope favors these procedures. To completely resect right hemi-colon, adequate exposure of superior mesenteric vein should be performed.
The main indications are introduced as follows: the depth of tumor invasion is T2–T3. The described procedure is suitable for early colon tumor and benign disease, and the elaborate preoperative examinations, such as CT scan and ultrasonic endoscopy, are necessary. The size of tumor is a very significant index. We recommend the maximal diameter is less than 8 centimeters. For the local advanced tumors and excessively obese patients, it is hard to extract the specimen through the vagina.
The right colic artery may also arise from the ileocolic or middle colic arteries and is absent in 2–18% of specimens. It supplies the ascending colon and hepatic flexure through its ascending and descending branches, both of them joining with neighboring vessels to contribute to the marginal artery. Thus, we should be prudent to deal with the arteries.
During our surgeries we performed functional end-to-end anastomosis (FEEA). The FEEA is a side-to-side anastomosis, and follows the excision of the ileocecal site and the ascending colon using linear staplers. There are many theories to explain why FEEA should fare better, including the wider diameter, a reduction in intraluminal pressure, and less proximal ischemia. The literature seems to suggest that the FEEA has become the most commonly preferred technique in recent times.
Here we make a description of the basic characteristics of patients who received the surgeries (Table 1).
Table 1
| Characteristics | Outcome |
|---|---|
| Mean age | 65 |
| BMI (kg/m2) | 25.5 |
| TNM | |
| I | 2 |
| II | – |
| III | – |
| IV | – |
| Adenoma | 3 |
| Tumor size (cm) | 4.0 |
| Operation time (min) | 210.3 |
| Blood loss (mL) | 25.5 |
| Open surgery | 0 |
| Complications | 0 |
| Function recover time (hr) | 19.7 |
| Lymph nodes examined | 13.1 |
| Positive margin | 0 |
BMI, body mass index.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.09.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leroy J, Cahill RA, Perretta S, et al. Natural orifice translumenal endoscopic surgery (NOTES) applied totally to sigmoidectomy: an original technique with survival in a porcine model. Surg Endosc 2009;23:24-30. [Crossref] [PubMed]
- Whiteford MH, Denk PM, Swanström LL. Feasibility of radical sigmoid colectomy performed as natural orifice translumenal endoscopic surgery (NOTES) using transanal endoscopic microsurgery. Surg Endosc 2007;21:1870-4. [Crossref] [PubMed]
- Al-Akash M, Boyle E, Tanner WA N.O.T.E.S.. the progression of a novel and emerging technique. Surg Oncol 2009;18:95-103. [Crossref] [PubMed]
- Canes D, Desai MM, Aron M, et al. Transumbilical single-port surgery: evolution and current status. Eur Urol 2008;54:1020-9. [Crossref] [PubMed]
- Franklin ME Jr, Kelley H, Kelley M, et al. Transvaginal extraction of the specimen after total laparoscopic right hemicolectomy with intracorporeal anastomosis. Surg Laparosc Endosc Percutan Tech 2008;18:294-8. [Crossref] [PubMed]
- Gill IS, Cherullo EE, Meraney AM, et al. Vaginal extraction of the intact specimen following laparoscopic radical nephrectomy. J Urol 2002;167:238-41. [Crossref] [PubMed]
- Ooi BS, Quah HM, Fu CW, et al. Laparoscopic high anterior resection with natural orifice specimen extraction (NOSE) for early rectal cancer. Tech Coloproctol 2009;13:61-4. [Crossref] [PubMed]
- Choi GS, Park IJ, Kang BM, et al. A novel approach of robotic-assisted anterior resection with transanal or transvaginal retrieval of the specimen for colorectal cancer. Surg Endosc 2009;23:2831-5. [Crossref] [PubMed]
- Franklin ME Jr, Kelley H, Kelley M, et al. Transvaginal extraction of the specimen after total laparoscopic right hemicolectomy with intracorporeal anastomosis. Surg Laparosc Endosc Percutan Tech 2008;18:294-8. [Crossref] [PubMed]
- McKenzie S, Baek JH, Wakabayashi M, et al. Totally laparoscopic right colectomy with transvaginal specimen extraction: the authors' initial institutional experience. Surg Endosc 2010;24:2048-52. [Crossref] [PubMed]
- Zhao ZX, Wang GY, Chen YG, et al. Laparoscopic Right Hemicolectomy with Transvaginal Specimen Extraction. Chinese Journal of Colorectal Diseases 2015;1:134-9.
Cite this article as: Wang G, Hu H, Zhang Q, Liu Z, Chen Y, Wang X. Laparoscopic right hemicolectomy with transvaginal specimen extraction. Ann Laparosc Endosc Surg 2016;1:28.


