Minimally invasive surgical management of chylothorax complicating esophagectomy
Chylothorax is a rare complication of esophagectomy which may be associated with metabolic, immunologic, and respiratory impairment, and increased postoperative mortality. A recent systematic review including 9,794 patients submitted to esophagectomy for cancer during the past three decades showed a 2.6% incidence of chylothorax (range, 0.9–9%). Use of neoadjuvant therapy and squamous carcinoma type were the leading predisposing factors based on a Cox-regression analysis. Two-third of patients underwent reoperative surgery at an average of 23 days after esophagectomy, and the reported mortality rate at 30-day was 1.6% (1).
Surgical anatomy
Chyle flows from the bowel lymphatic vessels to the cisterna chyli, and then through the thoracic duct, to ultimately empty near the junction of the left internal jugular and subclavian veins in the neck. Between 1,500 and 2,000 mL of chyle is drained by the venous system every day. An iatrogenic lesion of the thoracic duct is apparently more frequent during a transthoracic rather than a transhiatal esophageal resection (2). In most individuals, the thoracic duct arises from the cisterna chyli; in the chest, the duct is located between the azygos vein and the aorta, posterior to the esophagus. On computed tomography the cisterna chyli may resemble a retro-crural lymph node; in about 15% of patients it may be visualized by magnetic resonance at the level of L1–L2 vertebral bodies (3). Early division of the thoracic duct into two or more branches is present in up to 40% of the cases, and also the intrathoracic course of the duct before entering the left subclavian vein is variable. Since iatrogenic injury of the thoracic duct is infrequently recognized at operation, primary duct ligation during trans-thoracic esophagectomy has been recommended in order to prevent accidental lesions and subsequent chylous fistula (4).
Diagnosis
The diagnosis of chylothorax can be straightforward based on the typical milky appearance of fluid in the chest drain. However, in the fasting postoperative patient at bedside, a high-volume output from the drain or a recurrent pleural effusion may be the only sign of chyle leak. In these patients, assessment of triglyceride levels in the pleural fluid and a cream test per os or through the nasogastric tube can confirm the diagnosis. When chylothorax is diagnosed after removal of the chest drain and return to oral feeding, the patient can present with dyspnea and pleural effusion with the typical milky appearance. More rarely, atypical clinical presentation with severe hemodynamic instability secondary to a mediastinal chylocele may occur (5).
Management
Early surgical reoperation in patients with chylothorax has been advocated to reduce postoperative morbidity and mortality. This strategy is based on the evidence that depletion of T cells may occur within a week of chyle drainage despite best supportive care (6). However, given the fact that a chylous fistula can heal spontaneously, a two-week wait and see policy and conservative therapy with total parenteral nutrition and pleural drainage appears to be justified in patients with a drain output of less than 1,000 mL per day (7,8). Continuous suction drainage is necessary to prevent lung collapse and fibrin formation, and to accurately measure the rate of leakage. A non-fat, high protein diet can also be effective in reducing chyle flow, but addition of medium-chain triglycerides is necessary (9). It has been shown that thoracic duct sealing occurs faster with total parenteral nutrition rather than with enteral nutrition (10). Somatostatin and its analogue octreotide have been used in conjunction with other modalities for the conservative management of chylothorax (11,12), but no conclusive evidence of efficacy has emerged.
In patients with failure of medical treatment, ligation of the thoracic duct is mandatory. A two week time interval before surgical reintervention is reasonable to allow healing of the intrathoracic anastomosis and possibly reduce the trauma of an early trans-thoracic reoperation. A gastrografin swallow study or an upper digestive endoscopy should be performed before reoperation to make sure that no concomitant anastomotic problems exist that may need to be addressed at the same time. The classical surgical approach for ligation of the thoracic duct is through a right thoracotomy (13). Occasionally, a direct transabdominal approach to the cisterna chyli can be considered. Cream should be administered per os or through a nasogastric tube 4–6 hours before surgery to identify the site of the leak. If the duct is not clearly visible, mass ligation of the tissue between the aorta, azygos vein and spine should be performed.
Lampson (14) first showed that chylothorax could be controlled by supra-diaphragmatic ligation of the thoracic duct. Brinkmann and colleagues recently reported on 906 patients undergoing transthoracic esophageal resection and routine duct ligation at a single institution. Chylothorax occurred in 17 (1.9%) patients, and 15 of them underwent rethoracotomy and repeat duct ligation. Conservative therapy was preferred for two patients with a leak below 1,000 mL per day. Two deaths occurred among patients undergoing reoperation, giving an overall 90-day mortality of 11.7% (15).
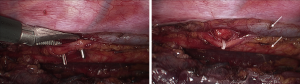
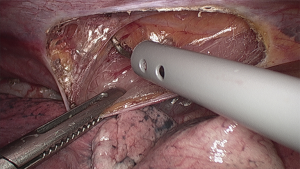
Despite the satisfactory outcomes and the marked reduction of hospital mortality, the propensity to early surgical reintervention in patients with chylothorax has been tempered in the past by the morbidity associated with thoracotomy. The advent of video-assisted thoracic surgery has offered a safe and effective therapeutic alternative in these patients. The thoracoscopic procedure for duct ligation can be performed in the left lateral decubitus using an angled 30-degree scope and three trocars. More recently, the thoracoscopic approach has been successfully performed also in the prone or semi-prone decubitus position (16) (Figure 1). After carefully pushing the gastric tube away from the spine, the injured duct releasing chyle in the posterior costophrenic angle is identified. Clips and sutures can be initially used to seal the thoracic duct, but occasionally this may prove unsuccessful (Figure 2). An alternative and safe approach consists of encircling en bloc the azygos vein and the periaortic fat tissue between the spine and the gastric tube using a conventional right-angled clamp. Subsequent application of a single blue cartridge of an articulating endostapler is generally effective in sealing the leak (2) (Figure 3).
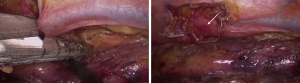
In patients in whom previous transthoracic attempts at duct ligation have failed, a direct transabdominal approach to the cisterna chyli through laparotomy or laparoscopy can be a reasonable option (5,17,18). The gastric tube crossing the diaphragmatic hiatus should be displaced toward the left side, and multiple suture ligations of the cisterna chyli and the origin of the thoracic duct at its origin are performed transhiatally along the right side of the aorta.
In conclusion, routine thoracic duct ligation should be performed at the time of trans-thoracic esophagectomy in an attempt to decrease the incidence of chylothorax. Reoperation for chyle leakage after esophagectomy should be performed early in the postoperative period since a prolonged conservative strategy is unlikely to be successful in the majority of patients and may be life-threatening. We recommend primary stapling of the thoracic duct en-bloc with the periaortic tissue through a right thoracoscopic approach in the semi-prone position.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.11.24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kranzfelder M, Gertler R, Hapfelmeier A, et al. Chylothorax after esophagectomy for cancer: impact of the surgical approach and neoadjuvant treatment: systematic review and institutional analysis. Surg Endosc 2013;27:3530-8. [Crossref] [PubMed]
- Bonavina L, Saino G, Bona D, et al. Thoracoscopic management of chylothorax complicating esophagectomy. J Laparoendosc Adv Surg Tech A 2001;11:367-9. [Crossref] [PubMed]
- Pinto PS, Sirlin CB, Andrade-Barreto OA, et al. Cisterna chyli at routine abdominal MR imaging: a normal anatomic structure in the retrocrural space. Radiographics 2004;24:809-17. [Crossref] [PubMed]
- Lai FC, Chen L, Tu YR, et al. Prevention of chylothorax complicating extensive esophageal resection by mass ligation of thoracic duct: a random control study. Ann Thorac Surg 2011;91:1770-4. [Crossref] [PubMed]
- Rottoli M, Russo IS, Bernardi D, et al. Atypical presentation and transabdominal treatment of chylothorax complicating esophagectomy for cancer. J Cardiothorac Surg 2012;7:9. [Crossref] [PubMed]
- Dugue L, Sauvanet A, Farges O, et al. Output of chyle as an indicator of treatment for chylothorax complicating oesophagectomy. Br J Surg 1998;85:1147-9. [Crossref] [PubMed]
- Breaux JR, Marks C. Chylothorax causing reversible T-cell depletion. J Trauma 1988;28:705-7. [Crossref] [PubMed]
- Merrigan BA, Winter DC, O'Sullivan GC. Chylothorax. Br J Surg 1997;84:15-20. [Crossref] [PubMed]
- Hashim SA, Roholt HB, Babayan VK, et al. Treatment of chyluria and chylothorax with medium-chain triglyceride. N Engl J Med 1964;270:756-61. [Crossref] [PubMed]
- Ramos W, Faintuch J. Nutritional management of thoracic duct fistulas. A comparative study of parenteral versus enteral nutrition. JPEN J Parenter Enteral Nutr 1986;10:519-21. [Crossref] [PubMed]
- Kelly RF, Shumway SJ. Conservative management of postoperative chylothorax using somatostatin. Ann Thorac Surg 2000;69:1944-5. [Crossref] [PubMed]
- Demos NJ, Kozel J, Scerbo JE. Somatostatin in the treatment of chylothorax. Chest 2001;119:964-6. [Crossref] [PubMed]
- Cerfolio RJ, Allen MS, Deschamps C, et al. Postoperative chylothorax. J Thorac Cardiovasc Surg 1996;112:1361-5; discussion 1365-6. [Crossref] [PubMed]
- Lampson RS. Traumatic chylothorax; a review of the literature and report of a case treated by mediastinal ligation of the thoracic duct. J Thorac Surg 1948;17:778-91. [PubMed]
- Brinkmann S, Schroeder W, Junggeburth K, et al. Incidence and management of chylothorax after Ivor Lewis esophagectomy for cancer of the esophagus. J Thorac Cardiovasc Surg 2016;151:1398-404. [Crossref] [PubMed]
- Bonavina L, Laface L, Abate E, et al. Comparison of ventilation and cardiovascular parameters between prone thoracoscopic and Ivor Lewis esophagectomy. Updates Surg 2012;64:81-5. [Crossref] [PubMed]
- Mason PF, Ragoowansi RH, Thorpe JA. Post-thoracotomy chylothorax--a cure in the abdomen? Eur J Cardiothorac Surg 1997;11:567-70. [Crossref] [PubMed]
- Icaza OJ Jr, Andrews K, Kuhnke M. Laparoscopic ligation of the thoracic duct in management of chylothorax. J Laparoendosc Adv Surg Tech A 2002;12:129-33. [Crossref] [PubMed]
Cite this article as: Asti E, Siboni S, Bonavina L. Minimally invasive surgical management of chylothorax complicating esophagectomy. Ann Laparosc Endosc Surg 2016;1:42.