Laparoscopic endoscopic cooperative surgery for early gastric cancer
Introduction
The laparoscopic and endoscopic cooperative surgery (LECS) was developed by Hiki et al. (1) as a surgical procedure for submucosal tumor (SMT), including the gastrointestinal stromal tumor (GIST), and is becoming popular across Japan. In the original procedure of the LECS, stomach wall is opened for resection of the tumor and the lumen is exposed to intraperitoneal space. Thus, the surgical procedure is not applicable for lesions with tumor exposed on the mucosal surface.
In order to address this problem, non-exposed endoscopic wall-inversion surgery (NEWS) (2), combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique (CLEAN-NET) (3), and closed LECS (4) have been developed as surgical procedures that do not require opening of the stomach wall. Furthermore, inverted LECS (5), which still needs opening of the stomach wall, has been developed as a surgical procedure avoiding spread of the tumor to the peritoneum.
In this article, we describe surgical procedures of closed LECS that we have applied in early gastric cancer cases. Additionally, we discuss tips and challenges involved.
Indications and principles
The basic indication is the early gastric cancer where endoscopic submucosal dissection (ESD) is not applicable. On the other hand, the LECS, regardless of open or closed procedures, cannot be applied to early gastric cancer unless omission of the lymph node dissection is not allowed since it is a surgical procedure that involves minimum partial resection of the stomach. Therefore, we routinely combine partial resection (closed LECS) with sentinel lymphatic basin (LB) dissection.
At present, our hospital has participated in “A study of Sentinel Node Navigation Surgery for Early Gastric Cancer” (6,7), which was approved as the advanced medical treatment (B). As a part of the clinical study, we conduct sentinel node navigation surgeries (SNNS) for cT1N0 early gastric cancer with a diameter of ≤4 cm. Additionally, we conduct this surgical procedure based on the distribution of lymph flow and the evaluation of rapid pathological diagnosis of the sentinel node (SN).
Preparations prior to the surgery
Radioisotope (RI) is locally injected in the evening one day before the surgery. The technetium-99m tin colloid (99mTc) is used as the tracer of the SNNS. Endoscopic local injections of 99mTc (0.5 mL) are made at four points close to the lesion.
Setup
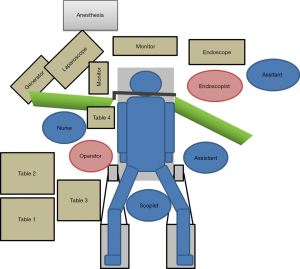
The patient’s posture and the preparation of surgical instruments correspond to those in regular laparoscopic distal gastrectomy (LDG). In the LECS, a monitor and tower for the oral endoscope are additionally used together with the instruments used for the regular LDG (Figure 1). If it is possible, the patient’s arms should be placed along the body so that the endoscope-set can be placed based on discussion with the anesthesiologist and operating room nurse.
Surgical technique
Insertion of port, pneumoperitoneum, and retraction of the liver
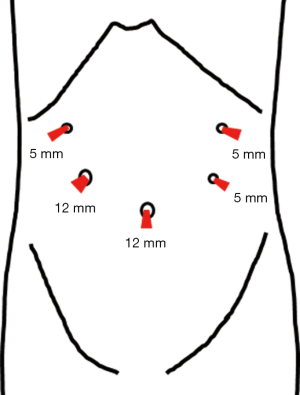
The position of the port corresponds to that during the regular LDG (Figure 2). By applying the concept of reduced port surgery (RPS) to the LECS, we sometimes reduce the number and/or the size of the port. If it is the case, we reduce the size of the port on the bottom-left and the top-right by 2–3 mm, from 5 to 3 or 2 mm. Unlike the regular LECS, when the LECS accompanies the lymph node dissection, it is important to introduce the RPS depending on the skills of the surgical team or the surgeon.
The observations of the peritoneum and the stomach are conducted under the pneumoperitoneum (10 mmHg). When the SNNS is performed, in order to observe lymph flow, in principle, the left lobe of the liver is retracted to the ventral side to ensure vision. We retract the liver by hanging a Silicon discTM (Hakko Co. Ltd., Nagano) percutaneously using the 2-0 Nylon straight needle (Figure 3). After finishing the observation of the peritoneum and retraction of the left lobe of the liver, the endoscopist starts to observe the inside of the stomach.
Marking and local injection of indocyanine green (ICG)
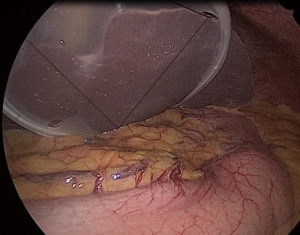
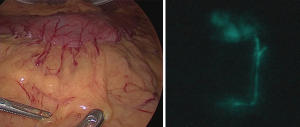
Marking is performed around the mucous membrane during oral endoscopic observation. Then, a local injection of 0.5 mL ICG 0.5% (5 mg/mL) is performed at four points around the major and minor axis directions (Figure 4). Special attention is required not to inject into the subserosal or extraserosal areas. When the serosa is observed during laparoscopy, lymph flow can be observed timely after the injection (Figure 5). For the observation, the laparoscopic system with which ICG fluorescence can be visually observed is used. In our department, the ICG-induced endoscopic fluorescence imaging system (SPIESTM) from KARL STORZ Inc. is used.
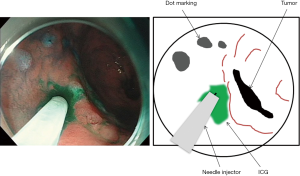
Sentinel lymph node drainage basin excision
The SN is the lymph node that is reached by ICG 15 min after local injection. Following this, the LB, including the SN, is dissected. When it is difficult to reduce the range of gastrectomy due to the relationship between the location of the LB and the site of the primary tumor, dissection of the lymph node above the LB and conventional gastrectomy (pylorus side gastrectomy or total gastrectomy) are performed. Thus, when the closed LECS is indicated, it is presumed that among the five regions that were classified based on the dominant region of five gastric arteries (8), it is limited to at most 1 or 2 adjacent areas.
Following removal of the whole SN using fluorescent endoscope system and gamma glove to detect the RI on the back table, the removed LB is sent for the intraoperative pathology diagnosis. The closed LECS is started when all examinations confirm negative SN.
Peripheral incision of mucosal/submucosal layers
The oral endoscope operation is resumed. The procedure corresponds to the regular LECS; a mucosal incision is made to connect the dot marking that is already placed on the mucosal surface around the tumor. Physiological saline is constantly injected under the mucous membrane of the incision, and the submucosa is peeled and dissected circumferentially in order to prevent damage of the serosal muscular layer (Figure 6). In order to prevent halation due to the laparoscope on the endoscope side, the level of the light source is minimized within the range where the abdominal cavity can be observed. The pneumoperitoneum pressure is reduced so that the endoscope can sufficiently supply air to the stomach (during intraperitoneal operation: approximately 10 mmHg→during operation of the endoscope: approximately 4 mmHg). It is also important to share information about the outside of the stomach wall (e.g., inadvertent puncture) with endoscopists by laparoscopy even when the mucosal/submucosal layers are circumferentially incised.
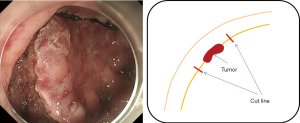
When targeting the lesion on the lesser curvature of the stomach, the angle of the endoscope differs depending on the location of the lesion (i.e., oral or anal side from the ventricular angle); regardless of whether looking down or reversing, a circumferential incision is made while ensuring the minimum necessary margin of about 1 cm from the tumor. Special attention should be paid not to perforate the stomach wall until a full circumference incision is completed.
Serosal marking and internal treatment
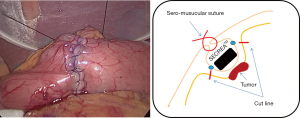
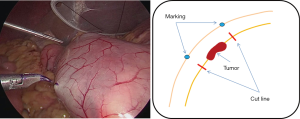
Marking is made endoscopically during laparoscopy using pyocyanine along the dissection line of the circumferential incision on the submucosal layer (Figure 7). At this time, by pushing the incision line with an endoscopic biopsy forceps, it is possible to confirm the line accurately and easily. A sponge material spacer that is adjusted to the size of the marking line is prepared. We use SECUREARTM (Hogy medical Co., Ltd). The SECUREATM is applied to the serosal surface of the tumor, and serosal faces are first sewn together at the central part to fix the SECUREATM (Figure 8). Thereafter, serosal muscular layer suture is evenly added until the SECUREATM is completely buried (Figure 9).
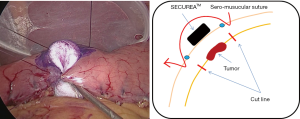
Complete resection of the tumor and endoscopic excision
The oral endoscopic operation is resumed, and incision of the muscular layer/serosa of the bulging tumor in which the SECUREATM has been inverted is made. The incision surface is tensed by itself due to the SECUREATM, and the SECUREATM is easily exposed after the full layer incision (Figure 10); therefore, it is possible to incise to the serosa accurately. The resected specimen is collected orally with an endoscopic retrieval device (Figure 11). It is not necessary to close the excised surface since the excised surface is endoscopically tightly sutured to the serosal muscle layer. When a mucosal surface defect is a concern, it can be sutured using the endoscopic clip. After carefully confirming the operation field both endoscopically and laparoscopically, the operation is ended. Nasogastric tubes and intraperitoneal drains are not routinely placed.
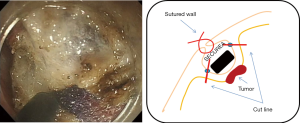
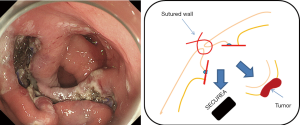
Postoperative management
The proton pump inhibitor (PPI) is administered after the surgery. Oral ingestion starts with water on the 2nd day according to the laparoscopic gastrectomy pass, and food is introduced and gradually increased in amount. Discharge is permitted at around the 8th day after the surgery. The patient visits the clinic about 1 month after the surgery and oral administration of PPI is continued until then. An endoscopic examination is performed at 3 months (Figure 12), 6 months, and 1 year after the surgery to observe the suture and presence of food debris. When gastrointestinal symptoms or excretory dysfunctions are found, stomachic medicine is prescribed.
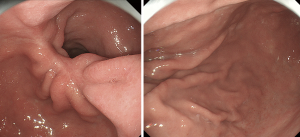
Conclusions
The procedure of the closed LECS itself is relatively easy and can be introduced to facilities where the LECS is already implemented. However, the challenges of the LECS for early gastric cancer that need to be addressed are (I) not to open the stomach wall during resection of the lesion area; and (II) to ensure correspondence to the lymph node metastasis. The LECS and the SNNS are the solutions for the former and latter problems, respectively. The combination of both techniques, while applicable cases are limited, is expected to be established as the effective surgical procedure of partial gastrectomy for early gastric cancer. In the future, we will develop the procedure carefully and proactively.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.01.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 2008;22:1729-35. [Crossref] [PubMed]
- Goto O, Mitsui T, Fujishiro M, et al. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer 2011;14:183-7. [Crossref] [PubMed]
- Inoue H, Ikeda H, Hosoya T, et al. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am 2012;21:129-40. [Crossref] [PubMed]
- Kikuchi S, Nishizaki M, Kuroda S, et al. Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Nunobe S, Hiki N, Gotoda T, et al. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer 2012;15:338-42. [Crossref] [PubMed]
- Takeuchi H, Kitagawa Y. New sentinel node mapping technologies for early gastric cancer. Ann Surg Oncol 2013;20:522-32. [Crossref] [PubMed]
- Kitagawa Y, Takeuchi H, Takagi Y, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol 2013;31:3704-10. [Crossref] [PubMed]
- Kinami S, Fujimura T, Ojima E, et al. PTD classification: proposal for a new classification of gastric cancer location based on physiological lymphatic flow. Int J Clin Oncol 2008;13:320-9. [Crossref] [PubMed]
Cite this article as: Inaki N, Moriyama H, Tsuji T. Laparoscopic endoscopic cooperative surgery for early gastric cancer. Ann Laparosc Endosc Surg 2017;2:22.