
Laparoscopic cholecystectomy and cirrhosis: patient selection and technical considerations
Introduction
The cholecystectomy is the most common elective abdominal surgical procedure performed worldwide, in the USA with more than 750,000 cases occurring each year (1). Patients’ comorbidities are known to increase resource utilization after both laparoscopic and open cholecystectomy (OC) (2). One major factor responsible for morbidity and mortality during cholecystectomy is liver cirrhosis. Even though cholecystectomy, umbilical and inguinal hernia repair have been shown to be the most secure non-hepatic surgery in cirrhotics patients, proper patient selection is paramount for favorable outcome (3).
The incidence of cholelithiasis in cirrhotic patients is higher than in general population, with a reported incidence of 9.5% to 13.7% versus 5.2% (4,5). The prevalence of gallstones in autopsy records varies from 29.4% to 46% in cirrhotics patients compared to around 13% in patients without liver disease (6,7). The reasons for this higher incidence include: decreased bile salt production, increased estrogen levels in cirrhotics patients, reduction in gallbladder motility and emptying and higher levels of unconjugated bilirubin, which are resultant from intravascular hemolysis and functional gallbladder alterations (8).
In the past, OC was the standard approach for patients requiring cholecystectomy. However laparoscopic cholecystectomy (LC) was introduced in 1980’s and demonstrated in non-cirrhotic patients substantial advantages over OC providing shorter convalescence period and hospital stay (9). Therefore, LC became the standard approach to most cholecystectomies (10). Nevertheless, initially discouraging results had been reported for biliary surgery in liver cirrhosis and portal hypertension patients: some series described morbidity and mortality rates as high as 23% and 25% (11-13). The main causes for these adverse results were excessive blood loss, renal failure, sepsis and postoperative liver failure. During the dissemination of LC technique, concerns were raised about the safety of performing LC in cirrhotics patients due to these unfavorable outcomes. Moreover, there was a general belief that the advantages of a minimally invasive procedure would be offset by the greater risk for complications associated with the lesser degree of tactile control (14) and three-dimensional feedback (15). These factors associated with possible hemorrhage related to portal hypertension, coagulopathy and thrombocytopenia, as well as the presence of abdominal adhesions and the reduced compliance of the fibrotic liver, which might impair the exposure of Callot’s triangle laparoscopically (16), would jeopardize the patient leading to poor outcomes. This led the NIH consensus statement of 1992 to publish that end-stage liver disease was a contra-indication to LC (17).
Nonetheless, LC became to be gradually performed in cirrhotic patients due more experience gained in minimally invasive surgery and the development of new surgical devices. In 1990s, the first studies addressing encouraging outcomes of LC in cirrhotic patients were published (18,19) and since then many case series have been reported, showing good results for LC in patients with liver cirrhosis. Still, no uniform consensus has been established in the surgical community regarding the most adequate procedure for treatment of gallstones in cirrhotic patients. The aim of this article is to summarize current trends of LC in cirrhotic patients, focusing in patient selection and technical considerations to improve outcomes.
Risk factors for surgical procedures in cirrhotics
Patients with cirrhosis undergoing non-hepatic surgery have a reported mortality in literature that may be as high as 45% (20). Historically, surgeons were often reluctant to operate on cirrhotics patients due to the possibility of developing acute on chronic liver failure, especially because anesthesia and surgical trauma. The use of less hepatotoxic regimens has suppressed this anesthetically concern (21). Variables traditionally related to increased morbidity-mortality in cirrhotic patients include: emergency surgery, Child-Pugh class C, presence of portal hypertension, ascites, encephalopathy, infection, anemia, malnutrition, jaundice, hypoalbuminemia, hypoxemia and prothrombin time (PT) not amendable by vitamin K or fresh-frozen plasma transfusion (21,22). Mortality rates of cholecystectomy in the setting of cirrhosis is linked to severity of underlying liver disease (23). However, the three variables identified in multivariated analysis to independently predict mortality after major surgical procedures in a large case-control study were age, the American Society of Anesthesiologists (ASA) Physical Status classification and Model of End Stage Liver Disease (MELD) score, especially when higher than 8 (24). A retrospective series addressing mortality specifically for LC in 220 cirrhotic patients identified these same three variables, with the cut-off for MELD score of 13 (25). Only Child-Pugh class A and B patients were included in this study. Another retrospective analysis of 94 cirrhotic patients who underwent LC found increased number of intraoperative transfusions of packed red blood cells to predict 30-day mortality on multivariated analysis (26).
LC in cirrhotics versus non-cirrhotic patients
The performance of gastrointestinal surgery procedures in cirrhotics patients is well-known to be associated with higher technical difficulty and increased morbidity-mortality. Cirrhosis is a major key intraoperative finding that contributes to surgical difficulty in LC (27). In fact, cirrhosis is one of the comorbidities most associated with postoperative resource utilization after cholecystectomy (2). A meta-analysis of six studies comparing LC in cirrhotics and non-cirrhotic patients reported a higher conversion rate to open procedure [7.06% versus 3.64%, P=0.0237, odds ratio (OR) =2.008, 95% confidence interval (CI): 1.085–3.718]; longer operative time (98.2 versus 70 minutes, P=0.00467, 95% CI: 8.93–47.47); increased intraoperative bleeding complications (26.4% versus 3.1%, P=0.001, OR =11.226, 95% CI: 7.022–17.946); increased estimated blood loss (105.6 versus 35.2 mL); and increased overall morbidity (20.86% versus 7.99%, P=0.001, OR =3.034, 95% CI: 1.958–4.702) (28). No statistically significant difference was found regarding wound infection incidence and death. Length of hospital stay was 5.4 versus 3.5 days, respectively (28). It is noteworthy nevertheless that more cirrhotics underwent an emergency procedure: acute cholecystitis was evident in 47% of patients with cirrhosis versus 14.7% of patients without cirrhosis (P=0.001, OR =5.141, 95% CI: 3.363–7.861). Emergency procedures in cirrhotics patients are associated with higher morbidity, longer postoperative hospitalization and a seven-fold higher mortality in comparison to elective surgery (29), which may result in bias when comparing these two groups. The overall poorer results of LC in cirrhotic patients comparing to non-cirrhotic ones should by no means preclude the utilization of minimally invasive surgery in cirrhotics patients.
LC versus OC
The majority of evidence in literature about LC and OC in cirrhotics patients comes from retrospective case series and retrospective case control studies. Some randomized clinical trials (RCT) have been produced (30-33) and were the subject of two recent meta-analysis (34,35). A systematic review identified 2,005 patients with cirrhosis undergoing LC (n=1,756) and OC (n=249) (34). General raw data showed that laparoscopic approach compared to OC was associated with fewer postoperative complications (17.6%×47.7%), fewer infectious complications (5.9%×19.9%), lower postoperative hepatic insufficiency rate (7.7%×18.1%) and lower mortality rate (0.8%×2%) (34). The conversion rate of LC was 5.8% and more patients were operated with acute cholecystitis in the OC group (19.6%×28.8%) (34). The prevalence of Child A, B and C patients were similar, being 76.6, 21.77 and 1.59% for LC and 67.2, 28.73 and 4.02 for OC, respectively (34). The meta-analysis of these RCT revealed the following advantages of LC: firstly, an overall lower postoperative complication rate (RR 0.52, 95% CI: 0.29–0.92; P=0.03) (35); secondly, lower incidence of infectious complications (OR 0.23, 95% CI: 0.10–0.56, P=0.001) (34); thirdly, shorter postoperative hospital stay (mean difference: 3.05 days, 95% CI: −4.09 to −2.01 days, P<0.001) (35); and finally, shorter time to resume a normal diet after LC (mean difference −27.48 hours; 95% CI: −30.96 to −23.99; P<0.001) (35). No statistically significant difference was observed in operation time, in number of blood transfusions required (35) and neither in postoperative hepatic insufficiency incidence (34). Overall mortality could not be compared due to lack of data in these RCTs. The most common complications of LC found in some large case series (22,36) (226 and 265 patients, respectively) include: postoperative worsening of ascites (10.6%), intraoperative bleeding (5.2%), intra-abdominal collections (3.5%) pulmonary infection (1.75%), blood transfusion (1.1%), recurrent stones in the gallbladder remnant after subtotal cholecystectomy (1.1%) and wound infection (1.1%).
Another meta-analysis of four case series comparing LC and OC found in the laparoscopic group (28): less operative blood loss (113 versus 425.2 mL, P=0.0154, 95% CI: 76.15–602.24); shorter operative time (123.3 versus 150.2 minutes, P=0.04173, 95% CI: 1.04–52.76) and decreased length of hospital stay (6 versus 12.2 days, P=0.001, 95% CI: 2.8–9.6). There was no statistically significant difference in morbidity (9.52% versus 15%, P=0.5922), mortality (4.76% versus 0%, P=0.99); or wound infection rates (0% versus 0.13%).
It is important to note that most of these data comes from Child-Pugh class A and B. In a large systematic review of literature including 1,310 cirrhotics patients undergoing LC (37), Machado et al. identified only 1.62% of patients as Child-Pugh class C, whereas class A accounted for 78.75% and class B, 19.5%. In the literature review of Laurence et al., only 1.59% and 4.02% of patients were Child C in LC and OC casuistic, respectively (34). As stated above, the overall mortality and mortality for cirrhotics patients undergoing LC is low, 0.45% and 17%, respectively (37). However, in Child-Pugh class C, mortality may be as high as 50% in individual series (25,38). Among the patients who died in Machado’s review (37), 17.1%, 0.97% and 0.12% were Child-Pugh class C, B and A, respectively. It is noteworthy that the mortality rates found in more modern series, for both LC and OC, are substantially lower than the ones reported in 1980s. This probably is the result of better patient selection, advances in critical care, more effective treatment of liver failure (including liver transplantation) and the availability of preoperative less invasive options, such as percutaneous cholecystostomy and endoscopic retrograde cholangiopancreatography (ERCP) (34). In the past, a significant portion of cirrhotics patients with jaundice underwent OC with open bile duct exploration and drainage procedures. Very often these explorations were not therapeutic, because the jaundice was due to chronic liver disease decompensation rather than obstruction (39,40). This scenario became almost extinct in the present days owing to the accuracy of imaging methods and efficacy of biliary endoscopic and percutaneous interventions (41,42). Another hallmark in cirrhosis medical approach was the improvement of liver transplantation throughout the 1980s, which revolutionized liver failure and portal hypertension management.
Another important aspect in the literature is that most procedures were elective. In the RCTs, they were 96% (35) and in overall LC in cirrhotics patients literature, 83% (37). There seems to be a higher incidence of acute cholecystitis in patients undergoing OC (34), although not statistically proven. This trend is also present in older reviews, in which indications for cholecystectomy were biliary colic (54%), acute cholecystitis (22%), asymptomatic gallstones (16%), acute pancreatitis (5%), acalculous cholecystitis (less than 1%) and cholangitis (less than 1%) (28).
The overall conversion for LC in cirrhotics patients ranges from 4.75% to 5.8% (34,37). Reasons for conversion include difficulty in identifying anatomy, uncontrolled bleeding, massive intracavitary adherences, the need of common bile duct exploration and suspected bile duct injury. It seems reasonable therefore that the MELD score has been recently shown to be an effective predictor for conversion, especially when higher than 14 (25,26). As conversion to OC is always an option, it seems intuitive to assume that morbidity will be lower than operating all cases with open technique, provided that minimal complications occur in the preliminary laparoscopic dissection. In fact, a population-based study from Chmielecki et al. (43), analyzing 2,857 LC and 383 OC in cirrhotics patients in USA, showed that even patients from converted LC had lower infection, transfusion, liver failure rates and similar reoperation rates to the OC group. One possible explanation is that the dissection done during LC may facilitate the further operative steps during OC. The total open operation may have a direct impact on the higher complication rate of OC (43). It should be highlighted that the conversion rate found in this study was 14% (43), higher than the found in most series (34,37). Still, this conversion rate is similar to other national inpatient study about acute cholecystitis (11%) (44). Interestingly, this study showed statistically significant lower mortality (1.3%×8.3%), lower ascites formation (9.7%×18%), lower liver failure rate (7%×1.4%), lower blood transfusion (7.4%×19.2%), lower postoperative haemorrhage (3.9%×6.6%) and lower postoperative infection rate (0.6%×3.5%) for LC and OC, respectively (43).
Special considerations in Child-Pugh class C patients
As previously mentioned, the literature addressing specifically Child-Pugh class C patients is scarce, as the casuistic of most studies are mainly composed of class A and, to a lesser extent, class B patients. As expected, class A patients presented decreased operative time, blood loss, conversion rate and lengthy of hospital stay compared to class B and C patients (45). Child C represents the end stage of the disease for cirrhotic patient; surgery is generally indicated only for lifesaving intervention. Few Child C patients are enrolled in trials or series reported in literature, and their outcomes are often combined with Child B patients. In a systematic review, Laurence et al. (34) identified 1,194 cirrhotic patients classified by Child-Pugh score who underwent LC, among them only 19 patients were Child C. Yet, when evaluating only the studies reporting mortality according to Child-Pugh score, they found 6 deaths, four Child C patients and the remaining two were Child A and B, respectively (34).
Morbidity and mortality in class C patients may reach up to 75% and 50%, respectively (38). Therefore, it is generally recommended that cholecystectomy should not be attempted in Child-Pugh class C cirrhotics patients unless they develop acute cholecystitis that does not respond to conservative medical management. These patients should be medically managed and downstaged prior to undergoing a cholecystectomy under non-emergent situations. It is also of utmost importance to consult the liver transplantation team preoperatively for all Child-Pugh class B and C cirrhotics patients in the event of postoperative decompensation or refer the patient to a tertiary care center (26). In this setting, patients with indication for liver transplantation should be kept in waiting list before surgery, since MELD allocation system allows to rescue these patients if liver function deteriorates. Whether or not LC is better than OC for this population is not clear due to the lack of data; however, based on the results for Child-Pugh class A and B patients, it seems safe to assume that a minimally invasive procedure is more advantageous provided that the surgeon has the required skills to do so.
A progressively more widespread approach for class C patients in emergency scenario includes the delayed cholecystectomy (46). In a retrospective analysis of acute severe cholecystitis in patients with advanced cirrhosis, Yao et al. (47) showed good results with ultrasound-guided percutaneous transhepatic cholecystostomy (PTC) and delayed LC. Successful performance of PTC readily relieves symptoms due to the rapid reduction of pressure within the gallbladder. The casuistic included 29 cirrhotics patients (6 Child-Pugh class A, 19 class B and 4 class C). The mean drainage treatment period was 8.1±1.4 days and mean hospitalization time was 15±7.2 days. All patients underwent LC, with no conversion to OC. Postoperative complications were fresh-frozen plasma transfusion (17.2%), postoperative worsening of ascites (3.45%) and postoperative deterioration in liver function tests (13.8%), which invariably recovered to baseline levels within 2 weeks. Thereby, we can infer that the results for delayed LC in this series were similar to the ones for up-front LC. Specific complications of PTC were puncture-related hemorrhage, which was drained via the catheter and spontaneously resolved (5.6%) and pain at the catheter site (37.9%), which could be medically managed. No bile leakage and no catheter dislodgement occurred. The authors concluded that PTC and delayed LC is a safe and effective procedure with minimal comorbidity for advanced cirrhotic patients presenting with acute cholecystitis (47). A similar approach may be implemented using biliary stenting of the cystic duct (48).
Advantages of LC in cirrhotics
Laparoscopic approach in cirrhotic patients has some extra advantages in comparison with non-cirrhotic patients (49). As a minimally invasive procedure, it ensures a quicker recovery and increases patients’ tolerability to the procedure. Moreover, it is safer for the surgical team, as many cirrhotics patients are infected with hepatitis B and C virus and there is less contact with patient’s blood and viscera. The access to the peritoneal cavity by millimetric channels reduces the incidence of ascites leak from surgical wound and the incidence of ascites infection, since it decreases inadvertent bacterial seeding and contamination of ascites. The magnification gained in laparoscopy allows the identification of twisted and dilated portal vein branches and of the congested gallbladder bed, avoiding thereby injury and bleeding. Additionally, laparoscopic technique imposes less abdominal wall trauma providing the preservation of abdominal wall collateral veins, avoiding bleeding during surgery and postoperative course. The positive abdominal pressure resultant from pneumoperitoneum also diminishes bleeding.
LC is associated with less peritoneal adhesions, which makes subsequent interventions easier and less traumatic. This is a valuable factor considering that several of these patients will be eligible for henceforth liver transplantation.
Technical aspects
The CO2 pneumoperitoneum can cause ischemia-reperfusion injury to the internal organs, which may aggravate damage to the hepatic function. That is the reason why it should be established with lower pressure and released slowly (50).
Bile spillage seems to be more common during LC than OC (21% versus 31%), respectively, however no postoperative problems are associated with it (51).
Uncontrolled bleeding may be one major problem during LC, and it is caused by abdominal varices and coagulopathy secondary to depressed clotting factor synthesis and thrombocytopenia. Therefore, fresh-frozen plasma and platelets may be given preoperatively as well as activated recombinant factor V11 (rFV11a) (52).
Preoperative imaging with computed tomography or magnetic resonance imaging is important, because it allows the identification of abdominal varices in abdominal wall and the potential presence of concurrent hepatoma (45). It is important to preserve the paraumbilical vein, since it is often recanalizated in patients with portal hypertension. Paraumbilical vein diverges part of the blood flow from the left portal branch to the systemic circulation, which keeps a faster blood flow in the portal trunk (53). Its patency has a substantial effect on portal pressure and can reduce the incidence of bleeding from the esophageal varices and increase the incidence of encephalopathy (54). Yet, paraumbilical interruption seems to be associated with postoperative portal vein thrombosis (55). The insertion of the first trocar should be infraumbilical using open technique to avoid damage to the umbilical and collateral veins (Figure 1).

The major difficulties encountered during LC in cirrhotic patients may be divided in five items (37):
- Adhesions with increased neovascularity;
- Difficult retraction of the liver;
- Inadequate exposure of the cholecystohepatic triangle;
- A high-risk gallbladder bed;
- A high-risk hilum.
Therefore, the following technical tips are recommended:
- Because of umbilical vein recanalization, the umbilical port should be made away from the falciform ligament, avoiding it completely.
- The liver parenchyma is hard and fibrotic and difficult to retract cranially. The retractor should be applied on the body of the gallbladder, just above the infundibulum, to expose the Callot’s triangle.
- Rotating all trocars positions to the right is useful, because when there is significant right hepatic lobe atrophy, the hepatic hilum structures tend to be twisted to the right side. Including one additional port site can help to expose the hilum, holding the left lobe or the duodenum. Using and aspirator in this port site is helpful to keep the surgical field clean and always ready to inject some irrigation for improving bipolar action.
- If these maneuvers do not allow correct exposure of the Callot’s triangle, then the fundus fist method should be used.
- The bipolar electrosurgery is really useful in surgeries of cirrhotic patients. It works much better than the monopolar instrument to coagulated bleedings in the gallbladder’s bed and hepatic parenchyma. Harmonic scalpel or advanced bipolar devices are also useful, especially for undoing peritoneal adhesions. Argon beam coagulation and thrombin spray may also be useful for hemostatic dissection. Blunt dissection should be kept at minimum.
- When regular surgical hemostatic steps failure to control oozing, there is the option of topical hemostatic agents, such as oxidized cellulose or hemostatic matrix sealant with thrombin and tranexamic acid. Finally, patience is crucial since conversion to OD may not always help to control the bleeding from coagulopathy.
- A subhepatic drain is usually placed because postoperative oozing is likely in the presence of coagulopathy. A closed drainage system is preferred to avoid ascites contamination. Drains are usually removed in 24 to 48 h.
- Consider laparoscopic needle biopsy of the liver to evaluate extent of cirrhosis and assist hepatologist team.
- Before completion of the procedure, all access ports should be checked for bleeding and all of them should be closed, even the 5 or 3 mm ones, because of postoperative ascites.
- The separation of the gallbladder from the liver bed may be difficult and the source of massive bleeding when tortuous, dilated vessels occur in the gallbladder bed. In such cases, the posterior wall may be left intact with the liver and the remnant mucosa is removed either by mucosectomy or by electrofulguration. This maneuver is called laparoscopic subtotal cholecystectomy (LSC) type I (36).
- Another variant of LSC, called LSC type II, consists of the circumferential division of the infundibulum as close as possible to the junction of the gallbladder and cystic duct, followed by the removal of the mucosa by mucosectomy or electrofulguration and closure of the stump by with harmonic scalpel or continuous polyglactin suture. It should be used when hilar dissection is difficult due to deeply placed hilum, inflammatory phlegmon, pericholecystic fibrosis or aberrant anatomy and vasculature in the cholecystohepatic triangle. These are more associated with a cavernamatous transformation of the portal vein. In patients with high-risk gallbladder bed and high-risk hilum, a combination of LSC I and II should be used, a variant that is called LSC type III.
In a series from Palanivelu et al. (36), LSC was used in 77.7% of 265 cirrhotics undergoing LC. Among these, 53.6% required an additional port and the fundus first technique was used in 8.3% of patients. Most LSC were type II (38.5%), followed by type I (23.4%) and type III (15.95%). The high LSC utilization in this series resulted in a low conversion rate (1%), but was associated with high postoperative bile leaks (52.8%). It was most common in patients undergoing LSC type II (94.1%) followed by LSC type I (38.1%). In fact, the major drawback of subtotal cholecystectomy, either via laparoscopy or open technique, is the bile leakage from the closed stump. The use of harmonic shears however is reported to markedly reduce this complication (56).
Final considerations
LC in cirrhotic patient remains a challenging operation that should be performed by surgeons with experience in both LC and the perioperative management of the cirrhotic patient. The increased morbidity-mortality in the cirrhotic population mandates two crucial principles to achieving favorable results with LC: careful patient selection and surgical technique modifications.
For elective and emergency cholecystectomy, Child-Pugh class A and B cirrhotics with MELD score lower than 18, LC is the optimal treatment. Class C patients in an elective scenario, on the other hand, should not be offered surgical treatment and instead be medically managed and downstaged. In an emergency setting, non-surgical temporization measures, such as PTC or endoscopic stenting of the cystic duct, should be first implemented to reduce the local inflammation and improve clinical condition, thereby allowing a safer and less morbid delayed LC. The liver transplantation team should also be consulted. This treatment algorithm is depicted in Figure 2.
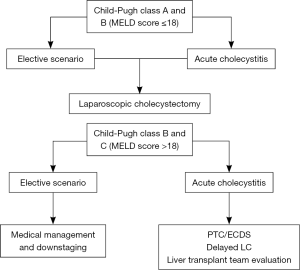
In summary, the past 1992 NIH consensus stating liver disease as a contra-indication for LC is undoubtedly no longer applicable to the present day. There is enough literature evidence to safely affirm that the cirrhotic population clearly benefits from a minimally invasive technique, achieving superior outcomes compared to open procedures, especially in terms of postoperative infection, liver function decompensation, intraoperative need of blood derivatives transfusion, lengthy of hospitalization, postoperative worsening of ascites, wound infection, incisional hernia incidence and mortality.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giovanni Battista Levi Sandri) for the series “Laparoscopic Liver Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.01.08). The series “Laparoscopic Liver Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Flum DR, Dellinger EP, Cheadle A, et al. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA 2003;289:1639-44. [Crossref] [PubMed]
- Boehme J, McKinley S, Michael Brunt L, et al. Patient comorbidities increase postoperative resource utilization after laparoscopic and open cholecystectomy. Surg Endosc 2016;30:2217-30. [Crossref] [PubMed]
- de Goede B, Klitsie PJ, Lange JF, et al. Morbidity and mortality related to non-hepatic surgery in patients with liver cirrhosis: a systematic review. Best Pract Res Clin Gastroenterol 2012;26:47-59. [Crossref] [PubMed]
- Bloch RS, Allaben RD, Walt AJ. Cholecystectomy in patients with cirrhosis. A surgical challenge. Arch Surg 1985;120:669-72. [Crossref] [PubMed]
- Genzini T, de Miranda MP. Cholelithiasis in cirrhotic patients. (Analysis of cholelithiasis among patients with liver cirrhosis in São Paulo, Brazil). Arq Gastroenterol 1996;33:52-9. [PubMed]
- Bouchier IA. Post mortem study of the frequency of gall- stones in patients with cirrhosis of the liver. Gut 1969;10:705-10. [Crossref] [PubMed]
- Iber FL, Caruso G, Polepalle C, et al. Increasing prevalence of gallstones in male veterans with alcoholic cirrhosis. Am J Gastroenterol 1990;85:1593-6. [PubMed]
- Keus F, de Jong JA, Gooszen HG, et al. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev 2006;CD006231 [PubMed]
- Angrisani L, Lorenzo M, Corcione F, et al. Gallstones in cirrhotics revisited by a laparoscopic view. J Laparoendosc Adv Surg Tech A 1997;7:213-20. [Crossref] [PubMed]
- Liguory C, Vitale GC. Biliary perestroika. Am J Surg 1990;160:237-8. [Crossref] [PubMed]
- Garrison RN, Cryer HM, Howard DA, et al. Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg 1984;199:648-55. [Crossref] [PubMed]
- Schwartz SI. Biliary tract surgery and cirrhosis: a critical combination. Surgery 1981;90:577-83. [PubMed]
- Aranha GV, Sontag SJ, Greenlee HB. Cholecystectomy in cirrhotic patients: a formidable operation. Am J Surg 1982;143:55-60. [Crossref] [PubMed]
- Salkini MW, Doarn CR, Kiehl N, et al. The role of haptic feedback in laparoscopic training using the LapMentor II. J Endourol 2010;24:99-102. [Crossref] [PubMed]
- Votanopoulos K, Brunicardi FC, Thornby J, et al. Impact of three-dimensional vision in laparoscopic training. World J Surg 2008;32:110-8. [Crossref] [PubMed]
- Lacy AM, Balaguer C, Andrade E, et al. Laparoscopic cholecystectomy in cirrhotic patients. Indication or contradiction? Surg Endosc 1995;9:407-8. [Crossref] [PubMed]
- Gallstones and laparoscopic cholecystectomy. NIH Consens Statement Online 1992;10:1-28. [PubMed]
- D'Albuquerque LA, de Miranda MP, Genzini T, et al. Laparoscopic cholecystectomy in cirrhotic patients. Surg Laparosc Endosc 1995;5:272-6. [PubMed]
- Yerdel MA, Tsuge H, Mimura H, et al. Laparoscopic cholecystectomy in cirrhotic patients: expanding indications. Surg Laparosc Endosc 1993;3:180-3. [PubMed]
- Demetriades D, Constantinou C, Salim A, et al. Liver cirrhosis in patients undergoing laparotomy for trauma: effect on outcomes. J Am Coll Surg 2004;199:538-42. [Crossref] [PubMed]
- Lopez-Delgado JC, Ballus J, Esteve F, et al. Outcomes of abdominal surgery in patients with liver cirrhosis. World J Gastroenterol 2016;22:2657-67. [Crossref] [PubMed]
- Yeh CN, Chen MF, Jan YY. Laparoscopic cholecystectomy in 226 cirrhotic patients. Experience of a single center in Taiwan. Surg Endosc 2002;16:1583-7. [Crossref] [PubMed]
- Mancero JM, D'Albuquerque LA, Gonzalez AM, et al. Laparoscopic cholecystectomy in cirrhotic patients with symptomatic cholelithiasis: a case-control study. World J Surg 2008;32:267-70. [Crossref] [PubMed]
- Teh SH, Nagorney DM, Stevens SR, et al. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology 2007;132:1261-9. [Crossref] [PubMed]
- Delis S, Bakoyiannis A, Madariaga J, et al. Laparoscopic cholecystectomy in cirrhotic patients: the value of MELD score and Child-Pugh classification in predicting outcome. Surg Endosc 2010;24:407-12. [Crossref] [PubMed]
- Quillin RC 3rd, Burns JM, Pineda JA. Laparoscopic cholecystectomy in the cirrhotic patient: predictors of outcome. Surgery 2013;153:634-40. [Crossref] [PubMed]
- Iwashita Y, Ohyama T, Honda G, et al. What are the appropriate indicators of surgical difficulty during laparoscopic cholecystectomy? Results from a Japan-Korea-Taiwan multinational survey. J Hepatobiliary Pancreat Sci 2016;23:533-47. [Crossref] [PubMed]
- Puggioni A, Wong LL. A metaanalysis of laparoscopic cholecystectomy in patients with cirrhosis. J Am Coll Surg 2003;197:921-6. [Crossref] [PubMed]
- Carbonell AM, Wolfe LG, DeMaria EJ. Poor outcomes in cirrhosis-associated hernia repair: a nationwide cohort study of 32,033 patients. Hernia 2005;9:353-7. [Crossref] [PubMed]
- Lausten SB, Ibrahim TM, El-Sefi T, et al. Systemic and cell-mediated immune response after laparoscopic and open cholecystectomy in patients with chronic liver disease. A randomized, prospective study. Dig Surg 1999;16:471-7. [Crossref] [PubMed]
- Ji W, Li LT, Wang ZM, et al. A randomized controlled trial of laparoscopic versus open cholecystectomy in patients with cirrhotic portal hypertension. World J Gastroenterol 2005;11:2513-7. [Crossref] [PubMed]
- El-Awadi S, El-Nakeeb A, Youssef T, et al. Laparoscopic versus open cholecystectomy in cirrhotic patients: a prospective randomized study. Int J Surg 2009;7:66-9. [Crossref] [PubMed]
- Hamad MA, Thabet M, Badawy A, et al. Laparoscopic versus open cholecystectomy in patients with liver cirrhosis: a prospective, randomized study. J Laparoendosc Adv Surg Tech A 2010;20:405-9. [Crossref] [PubMed]
- Laurence JM, Tran PD, Richardson AJ, et al. Laparoscopic or open cholecystectomy in cirrhosis: a systematic review of outcomes and meta-analysis of randomized trials. HPB (Oxford) 2012;14:153-61. [Crossref] [PubMed]
- de Goede B, Klitsie PJ, Hagen SM, et al. Meta-analysis of laparoscopic versus open cholecystectomy for patients with liver cirrhosis and symptomatic cholecystolithiasis. Br J Surg 2013;100:209-16. [Crossref] [PubMed]
- Palanivelu C, Rajan PS, Jani K, et al. Laparoscopic cholecystectomy in cirrhotic patients: the role of subtotal cholecystectomy and its variants. J Am Coll Surg 2006;203:145-51. [Crossref] [PubMed]
- Machado NO. Laparoscopic Cholecystectomy in Cirrhotics. JSLS 2012;16:392-400. [Crossref] [PubMed]
- Currò G, Iapichino G, Melita G, et al. Laparoscopic cholecystectomy in Child-Pugh class C cirrhotic patients. JSLS 2005;9:311-5. [PubMed]
- Isozaki H, Okajima K, Morita S, et al. Surgery for cholelithiasis in cirrhotic patients. Surg Today 1993;23:504-8. [Crossref] [PubMed]
- Cryer HM, Howard DA, Garrison RN. Liver cirrhosis and biliary surgery: assessment of risk. South Med J 1985;78:138-41. [Crossref] [PubMed]
- Berman M, Nudelman IL, Fuko Z, et al. Percutaneous transhepatic cholecystostomy: effective treatment of acute cholecystitis in high risk patients. Isr Med Assoc J 2002;4:331-3. [PubMed]
- Itoi T, Wang HP. Endoscopic management of bile duct stones. Dig Endosc 2010;22:S69-75. [Crossref] [PubMed]
- Chmielecki DK, Hagopian EJ, Kuo YH, et al. Laparoscopic cholecystectomy is the preferred approach in cirrhosis: a nationwide, population-based study. HPB (Oxford) 2012;14:848-53. [Crossref] [PubMed]
- Csikesz NG, Tseng JF, Shah SA. Trends in surgical management for acute cholecystitis. Surgery 2008;144:283-9. [Crossref] [PubMed]
- Nguyen KT, Kitisin K, Steel J, et al. Cirrhosis is not a contraindication to laparoscopic cholecystectomy: results and practical recommendations. HPB (Oxford) 2011;13:192-7. [Crossref] [PubMed]
- Currò G, Cucinotta E. Percutaneous gall bladder aspiration as an alternative to laparoscopic cholecystectomy in Child-Pugh C cirrhotic patients with acute cholecystitis. Gut 2006;55:898-9. [Crossref] [PubMed]
- Yao Z, Hu K, Huang P, et al. Delayed laparoscopic cholecystectomy is safe and effective for acute severe calculous cholecystitis in patients with advanced cirrhosis: a single center experience. Gastroenterol Res Pract 2014;2014:178908.
- Shrestha R, Trouillot TE, Everson GT. Endoscopic stenting of the gallbladder for symptomatic gallbladder disease in patients with end-stage liver disease awaiting orthotopic liver transplantation. Liver Transpl Surg 1999;5:275-81. [Crossref] [PubMed]
- Lledó JB, Ibañez JC, Mayor LG, et al. Laparoscopic cholecystectomy and liver cirrhosis. Surg Laparosc Endosc Percutan Tech 2011;21:391-5. [Crossref] [PubMed]
- Ozmen MM, Kessaf Aslar A, Besler HT, et al. Does splanchnic ischemia occur during laparoscopic cholecystectomy? Surg Endosc 2002;16:468-71. [Crossref] [PubMed]
- Poggio JL, Rowland CM, Gores GJ, et al. A comparison of laparoscopic and open cholecystectomy in patients with compensated cirrhosis and symptomatic gallstone disease. Surgery 2000;127:405-11. [Crossref] [PubMed]
- Jeffers L, Chalasani N, Balart L, et al. Safety and efficacy of recombinant factor VIIa in patients with liver disease undergoing laparoscopic liver biopsy. Gastroenterology 2002;123:118-26. [Crossref] [PubMed]
- Widman A, de Oliveira IR, Speranzini MB, et al. Patent paraumbilical vein: hemodynamic importance in mansonic hepatosplenic portal hypertension (Study with ultrasonography Doppler. Arq Gastroenterol 2001;38:221-6. [PubMed]
- Gupta D, Chawla YK, Dhiman RK, et al. Clinical significance of patent paraumbilical vein in patients with liver cirrhosis. Dig Dis Sci 2000;45:1861-4. [Crossref] [PubMed]
- Andraus W, Pinheiro RS, Haddad LB, et al. The best approach for splenectomy in portal hypertension. Surgery 2011;149:853. [Crossref] [PubMed]
- Bessa SS, Abdel-Razek AH, Sharaan MA, et al. Laparoscopic cholecystectomy in cirrhotics: a prospective randomized study comparing the conventional diathermy and the harmonic scalpel for gallbladder dissection. J Laparoendosc Adv Surg Tech A 2011;21:1-5. [Crossref] [PubMed]
Cite this article as: Pinheiro RS, Waisberg DR, Lai Q, Andraus W, Nacif LS, Rocha-Santos V, D’Albuquerque LAC. Laparoscopic cholecystectomy and cirrhosis: patient selection and technical considerations. Ann Laparosc Endosc Surg 2017;2:35.


