Secrets for successful laparoscopic antireflux surgery: mesh hiatoplasty
IntroductionOther Section
- Introduction
- The challenge of recurrence
- Use of mesh at the esophageal hiatus
- Surgical technique
- Tips, tricks and pitfalls
- Conclusions and future directions
- Acknowledgments
- Footnote
- References
The role of the esophageal hiatus in preventing gastro-esophageal reflux was first established in the mid-twentieth century by British surgeon Phillip Allison (1). Based on his observations, Allison devised an operation that would re-establish normal anatomy through suture of the diaphragmatic crura posterior to the esophagus and fixation of the cardia to the diaphragm on the abdominal side (2). During the same decade, Rudolph Nissen noted that plication of the fundus around the distal esophagus eliminated symptoms of gastro-esophageal reflux (3). These two concepts, closure of the hiatus and creation of a flap-valve mechanism, have since formed the basis of anti-reflux procedures.
Significant progress has been made in anti-reflux surgery since the time of Allison and Nissen. With the introduction of minimally invasive techniques to foregut surgery, patients no longer have to endure large abdominal or thoracic incisions. Recent population-based analysis has shown a decrease in the morbidity-mortality of hiatal hernia repair over the last 20 years, with the lowest risk in those treated by laparoscopy (4). Despite these advances, recurrence of hiatal hernias (with its associated risk of recurrent GERD) remains common (5). This fact has driven many to seek improvements in technique, notably by addition of prosthetic material (mesh) to the repair.
The challenge of recurrenceOther Section
- Introduction
- The challenge of recurrence
- Use of mesh at the esophageal hiatus
- Surgical technique
- Tips, tricks and pitfalls
- Conclusions and future directions
- Acknowledgments
- Footnote
- References
The esophageal hiatus is subject to constant diaphragmatic movement, positive pressure from the abdomen, and negative pressure from the mediastinum. These conditions set the stage for recurrence of hiatal or paraesophageal hernias after surgical repair. Indeed, the Allison procedure fell into disuse after he reported on a nearly 50% recurrence rate for hiatal hernia at the 1973 meeting of the American Surgical Association. Allison carefully documented recurrence as demonstrated by barium swallow studies, and was the first to point out that the rate of recurrence increases over time (6). Difficulties in hiatal hernia repair were not unique to the Allison technique, as evidenced by Ronald Belsey’s work. Belsey’s Mark IV repair, described in 1967, was the culmination of a technique that evolved (Marks I–III) over years of trial and error and methodical observation. Recurrence rate for the Mark IV repair was noted to be 7%, with 50% of patients having undergone barium swallow at least a year after their operation (7). Only Hill’s 1977 description of his median arcuate ligament repair stood out in reporting a 0% recurrence rate (8). However, there were few studies in the open era to track large scale outcomes, and even fewer that tracked anatomic ones. As such, subsequent reports of the Hill repair found recurrence nearing 20% in those who underwent radiologic follow-up (9).
The laparoscopic approach to hiatal hernia repair was first described in the early 1990’s (10). This approach offered patients the known advantages of minimally invasive surgery while allowing surgeons to follow the tenets of successful hiatal hernia repair, namely esophageal mobilization, excision of the hernia sac and closure of the diaphragmatic crura (11). With this renewed enthusiasm for hiatal hernia repair, came more studies evaluating outcomes and anatomic failure. Despite coming under fire initially for increased recurrence rate when compared to open surgery, the laparoscopic technique has continued to be favored by most high volume centers (12-14), as the outcomes now appear similar to open approaches.
Use of mesh at the esophageal hiatusOther Section
- Introduction
- The challenge of recurrence
- Use of mesh at the esophageal hiatus
- Surgical technique
- Tips, tricks and pitfalls
- Conclusions and future directions
- Acknowledgments
- Footnote
- References
Non-absorbable mesh
The success of prosthetic material for repair of abdominal wall hernias suggested that the same tension-free concept could be applied to the esophageal hiatus. Kuster and Gilroy first reported placement of mesh at the hiatus in 1993. In their technique, a polyester mesh was placed anterior to the esophagus as a bridge repair (15). Many others soon followed suit, with case series documenting the use of mesh (most commonly polypropylene and PTFE) placed in a variety of configurations, including O-shaped (encircling the esophagus), U-shaped, anteriorly placed triangle and posteriorly placed rectangle (16-19). These techniques were found to be associated with decreased recurrence (20,21). However, it was soon found that the use of non-absorbable mesh was linked to unique complications, of which the most troubling were esophageal stricture and erosion of mesh into the esophagus (22,23).
Some groups have reported low incidence of dysphagia and continue to advocate for the use of non-absorbable mesh at the hiatus (24-26). This remains a controversial topic as mesh erosion into the esophageal lumen has been associated with catastrophic consequences (i.e., need for esophago-gastrectomy) (19,22,27).
Absorbable mesh
In an effort to take advantage of the benefits of prosthetic material while avoiding complications associated with non-absorbable mesh, our group was an early adopter of the use of absorbable (biologic) mesh at the hiatus. After our initial results showed a promising safety profile and low recurrence rate (28), we designed a multicenter randomized controlled trial comparing sutured repair to closure reinforced with biologic mesh (small intestinal submucosa, Surgisis, Cook Surgical). At 6-month follow-up, we found that compared to suture repair, use of biologic mesh was associated with a nearly three-fold reduction in recurrence (9% vs. 24%) (29). These encouraging results were later tempered by our 5-year follow-up, in which we found similar recurrence rates between both groups (54% vs. 59%) (30). The outcomes of this study led us to take a selective approach to the placement of biologic mesh at the hiatus.
A recent report aimed to compare results over two types of biologic mesh (both composed of acellular human dermis) in 54 patients. Overall recurrence rate was 14% at 6-month follow-up and no significant differences were found between the two groups (31). A European observational study compared results between patients who underwent suture-only repair and repair buttressed with a biosynthetic mesh. Overall recurrence was higher in the suture-only group (9.7% vs. 18.6%) at a median follow up of 24 months. However, when recurrence-free probability was estimated, the benefit of mesh largely diminished over time (32). Thus results from these studies have largely confirmed our findings, i.e., placing biologic mesh is superior in terms of short-term recurrence, an advantage which decreases over time.
There are very few head-to-head studies comparing synthetic and biologic mesh at the hiatus. The one randomized controlled trial to do so found similar 6-month recurrence rates in three groups studied: suture only repair, biologic mesh repair and non-absorbable mesh repair. This study is ongoing and its long-term results should shed further light into the complication and recurrence profiles of each type of repair (33).
Surgical techniqueOther Section
- Introduction
- The challenge of recurrence
- Use of mesh at the esophageal hiatus
- Surgical technique
- Tips, tricks and pitfalls
- Conclusions and future directions
- Acknowledgments
- Footnote
- References
The patient is placed in a supine, split-leg position. Both arms are tucked and safety straps are placed around the patients’ thighs. The surgeon stands at the foot of the table, with an assistant standing on the patient’s left side.
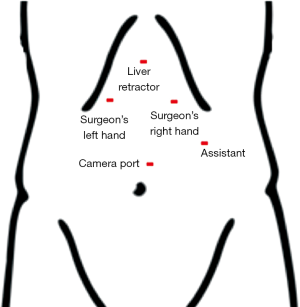
Pneumoperitoneum is obtained with a Veres needle placed through a left subcostal incision. We place our first trocar in the same location using an 11-mm optical port. We place three additional ports as seen in Figure 1. Additionally, a Nathanson liver retractor (Mediflex, Islandia, NY, USA) is placed in a subxiphoid location. A steep reverse Trendelenburg position allows visualization of the esophageal hiatus.
We start the procedure by reducing as much hernia contents as possible. If the stomach is not easily reducible, no further attempts are made at this point. An incision of the sac over the left crus is made, which will enhance visualization when completing ligation of the short gastric vessels. These are taken down using the Ligasure device (Covidien, Boulder, CO, USA). Once the fundus is completely free of attachments, the areolar plane between the hernia sac and the left crus is entered. At this point, adequate identification and dissection of planes is crucial as it allows better identification of mediastinal structures, including the pleura. From the left crus, dissection of the hernia sac is carried over to the right in a counterclockwise fashion. This usually allows for full reduction of hernia contents and sac. The anterior vagus is identified and protected before reduction of the hernia sac.
With the stomach in an intra-abdominal position, a Penrose drain is placed around the GE junction. This will allow traction of the esophagus during mediastinal mobilization. The esophagus can be readily mobilized from its mediastinal attachments with a combination of blunt and energy dissection. At least 3 cm of intra-abdominal esophagus should be obtained.
Closure of the hiatus is usually achieved through posterior 2-0 silk sutures. Anterior stitches may also be placed if the esophagus is deemed to be angulated by only posterior sutures. We do this over a 52-Fr lighted bougie.
We routinely perform a 360-degree Nissen fundoplication unless otherwise contraindicated. In patients with ineffective motility as demonstrated by high-resolution manometry, it is our preference to perform a partial (Toupet) fundoplication.
The difficult hiatal closure
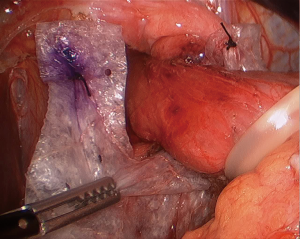
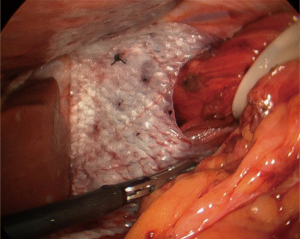
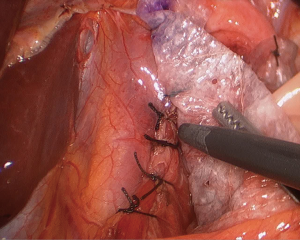
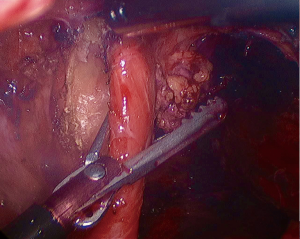
As previously mentioned, our approach to placing mesh is highly selective and the decision is mostly based on intraoperative findings. If the operating surgeon considers that the repair is being made under tension, a biologic mesh is placed to buttress the repair. We either cut the mesh in a “U” shape (Figure 2), which completely covers the hiatal closure, or in a “C” shape (Figure 3) to cover the posterior closure, anterior hiatus and right crus. The mesh is secured through superior sutures placed on both sides of the hiatus with additional fixation achieved by applying fibrin glue to the entire mesh (Figure 4).
In rare occasions, excessive tension at the hiatus will make it impossible or near impossible to adequately bring the crura together. In those instances, we perform a diaphragmatic relaxing incision on the right side. In this technique, a full thickness incision is made 2 cm lateral to the edge of the right crus (Figure 5). A biologic mesh is then placed in the “C” shape, which covers both the incision and the crural closure. The mesh is secured to the diaphragm with 4–5 stitches and fibrin glue.
Tips, tricks and pitfallsOther Section
- Introduction
- The challenge of recurrence
- Use of mesh at the esophageal hiatus
- Surgical technique
- Tips, tricks and pitfalls
- Conclusions and future directions
- Acknowledgments
- Footnote
- References
Use of tacks to for mesh fixation
Non-absorbable or absorbable tacks are widely used for mesh fixation in ventral and inguinal hernia repair. While there is concern for chronic pain and higher recurrence rate, tacks have been found to be at least as safe as suture fixation, and complications are rarely life threatening (34,35). The opposite is true in hiatal hernia repair. The reported rate of complications from tacks at the hiatus is low, however they are associated with grim complications such as pericardial injury and consequent cardiac tamponade. Various reported cases have only been diagnosed on autopsy (36-38). We therefore advocate for extreme care when suturing mesh at the diaphragm (taking very superficial bites just to encourage scarring to the diaphragm) and avoiding fixation by tacks at all costs.
Conclusions and future directionsOther Section
- Introduction
- The challenge of recurrence
- Use of mesh at the esophageal hiatus
- Surgical technique
- Tips, tricks and pitfalls
- Conclusions and future directions
- Acknowledgments
- Footnote
- References
While preventing recurrences is a major point of emphasis for hiatal hernia repair, the success of prosthetic material for abdominal wall surgery has not been duplicated for operations at the hiatus. The incompletely realized quest for reduction of complications and recurrence will likely drive many improvements in the years to come. Current evidence supports the use of absorbable mesh to reduce short-term recurrence, though strategies for reducing long-term recurrences are lacking.
AcknowledgmentsOther Section
- Introduction
- The challenge of recurrence
- Use of mesh at the esophageal hiatus
- Surgical technique
- Tips, tricks and pitfalls
- Conclusions and future directions
- Acknowledgments
- Footnote
- References
Funding: None.
FootnoteOther Section
- Introduction
- The challenge of recurrence
- Use of mesh at the esophageal hiatus
- Surgical technique
- Tips, tricks and pitfalls
- Conclusions and future directions
- Acknowledgments
- Footnote
- References
Provenance and Peer Review: This article was commissioned by the Guest Editor (Fernando A. M. Herbella) for the series “Secrets for Successful Laparoscopic Antireflux Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.02.16). The series “Secrets for Successful Laparoscopic Antireflux Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- The challenge of recurrence
- Use of mesh at the esophageal hiatus
- Surgical technique
- Tips, tricks and pitfalls
- Conclusions and future directions
- Acknowledgments
- Footnote
- References
- Stylopoulos N, Rattner DW. The history of hiatal hernia surgery: from Bowditch to laparoscopy. Ann Surg 2005;241:185-93. [PubMed]
- Allison PR. Reflux esophagitis, sliding hiatal hernia, and the anatomy of repair. Surg Gynecol Obstet 1951;92:419-31. [PubMed]
- Nissen R. Eine einfache operation zur beeinflussung der refluxoesophagitis. Schweiz Med Wochenschr 1956;86:590-2. [PubMed]
- Kaplan JA, Schecter S, Lin MY, et al. Morbidity and Mortality Associated With Elective or Emergency Paraesophageal Hernia Repair. JAMA Surg 2015;150:1094. [Crossref] [PubMed]
- Tam V, Winger DG, Nason KS. A systematic review and meta-analysis of mesh vs suture cruroplasty in laparoscopic large hiatal hernia repair. Am J Surg 2016;211:226-38. [Crossref] [PubMed]
- Allison PR. Hiatus hernia: a 20-year retrospective survey. Ann Surg 1973;178:273-6. [Crossref] [PubMed]
- Skinner DB, Belsey RH. Surgical management of esophageal reflux and hiatus hernia. Long-term results with 1,030 patients. J Thorac Cardiovasc Surg 1967;53:33-54. [PubMed]
- Hill LD. Progress in the surgical management of hiatal hernia. World J Surg 1977;1:425-36. [Crossref] [PubMed]
- Aye RW, Rehse D, Blitz M, et al. The Hill antireflux repair at 5 institutions over 25 years. Am J Surg 2011;201:599-604. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Nathanson LK. Laparoscopic reduction, crural repair, and fundoplication of large hiatal hernia. Am J Surg 1992;163:425-30. [Crossref] [PubMed]
- Oddsdottir M, Franco AL, Laycock WS, et al. Laparoscopic repair of paraesophageal hernia. New access, old technique. Surg Endosc 1995;9:164-8. [Crossref] [PubMed]
- Mattar SG, Bowers SP, Galloway KD, et al. Long-term outcome of laparoscopic repair of paraesophageal hernia. Surg Endosc 2002;16:745-9. [Crossref] [PubMed]
- Levy G, Aye RW, Farivar AS, et al. A Combined Nissen Plus Hill Hybrid Repair for Paraesophageal Hernia Improves Clinical Outcomes and Reduces Long-Term Recurrences Compared with Laparoscopic Nissen Alone. J Gastrointest Surg 2017;21:121-5. [Crossref] [PubMed]
- Oelschlager BK, Petersen RP, Brunt LM, et al. Laparoscopic Paraesophageal Hernia Repair: Defining Long-Term Clinical and Anatomic Outcomes. J Gastrointest Surg 2012;16:453-9. [Crossref] [PubMed]
- Kuster GG, Gilroy S. Laparoscopic technique for repair of paraesophageal hiatal hernias. J Laparoendosc Surg 1993;3:331-8. [Crossref] [PubMed]
- Frantzides CT, Carlson MA. Prosthetic reinforcement of posterior cruroplasty during laparoscopic hiatal herniorrhaphy. Surg Endosc 1997;11:769-71. [Crossref] [PubMed]
- Edelman DS. Laparoscopic paraesophageal hernia repair with mesh. Surg Laparosc Endosc 1995;5:32-7. [PubMed]
- Pitcher DE, Curet MJ, Martin DT, et al. Successful laparoscopic repair of paraesophageal hernia. Arch Surg 1995;130:590-6. [Crossref] [PubMed]
- Targarona EM, Bendahan G, Balague C, et al. Mesh in the hiatus: a controversial issue. Arch Surg 2004;139:1286-96. [Crossref] [PubMed]
- Frantzides CT, Madan AK, Carlson MA, et al. A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg 2002;137:649-52. [Crossref] [PubMed]
- Granderath FA, Schweiger UM, Kamolz T, et al. Laparoscopic antireflux surgery with routine mesh-hiatoplasty in the treatment of gastroesophageal reflux disease. J Gastrointest Surg 2002;6:347-53. [Crossref] [PubMed]
- Tatum RP, Shalhub S, Oelschlager BK, et al. Complications of PTFE Mesh at the Diaphragmatic Hiatus. J Gastrointest Surg 2008;12:953-7. [Crossref] [PubMed]
- Stadlhuber RJ, Sherif AE, Mittal SK, et al. Mesh complications after prosthetic reinforcement of hiatal closure: a 28-case series. Surg Endosc 2009;23:1219-26. [Crossref] [PubMed]
- Granderath FA, Carlson MA, Champion JK, et al. Prosthetic closure of the esophageal hiatus in large hiatal hernia repair and laparoscopic antireflux surgery. Surg Endosc 2006;20:367-79. [Crossref] [PubMed]
- Antoniou SA, Antoniou GA, Koch OO, et al. Lower recurrence rates after mesh-reinforced versus simple hiatal hernia repair: a meta-analysis of randomized trials. Surg Laparosc Endosc Percutan Tech 2012;22:498-502. [Crossref] [PubMed]
- Müller-Stich BP, Linke GR, Senft J, et al. Laparoscopic Mesh-augmented Hiatoplasty With Cardiophrenicopexy Versus Laparoscopic Nissen Fundoplication for the Treatment of Gastroesophageal Reflux Disease. Ann Surg 2015;262:721-5; discussion 725-7. [Crossref] [PubMed]
- Priego P, Perez de Oteyza J, Galindo J, et al. Long-term results and complications related to Crurasoft® mesh repair for paraesophageal hiatal hernias. Hernia 2017;21:291-8. [PubMed]
- Oelschlager BK, Barreca M, Chang L, et al. The use of small intestine submucosa in the repair of paraesophageal hernias: initial observations of a new technique. Am J Surg 2003;186:4-8. [Crossref] [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter J, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg 2006;244:481-90. [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg 2011;213:461-8. [Crossref] [PubMed]
- Ward KC, Costello KP, Baalman S, et al. Effect of acellular human dermis buttress on laparoscopic hiatal hernia repair. Surg Endosc 2015;29:2291-7. [Crossref] [PubMed]
- Asti E, Lovece A, Bonavina L, et al. Laparoscopic management of large hiatus hernia: five-year cohort study and comparison of mesh-augmented versus standard crura repair. Surg Endosc 2016;30:5404-9. [Crossref] [PubMed]
- Watson DI, Thompson SK, Devitt PG, et al. Laparoscopic Repair of Very Large Hiatus Hernia With Sutures Versus Absorbable Mesh Versus Nonabsorbable Mesh. Ann Surg 2015;261:282-9. [Crossref] [PubMed]
- Kitamura RK, Choi J, Lynn E, et al. Suture versus tack fixation of mesh in laparoscopic umbilical hernia repair. JSLS 2013;17:560-4. [Crossref] [PubMed]
- Muysoms F, Vander Mijnsbrugge G, Pletinckx P, et al. Randomized clinical trial of mesh fixation with “double crown” versus ‘sutures and tackers’ in laparoscopic ventral hernia repair. Hernia 2013;17:603-12. [Crossref] [PubMed]
- Frantzides CT, Welle SN. Cardiac tamponade as a life-threatening complication in hernia repair. Surgery 2012;152:133-5. [Crossref] [PubMed]
- Thijssens K, Hoff C, Meyerink J. Tackers on the diaphragm. Lancet 2002;360:1586. [Crossref] [PubMed]
- McClellan JM, Nelson D, Martin M. Hemopericardium after laparoscopic perihiatal procedures: high index of suspicion facilitates early diagnosis and successful nonoperative management. Surg Obes Relat Dis 2016;12:e27-31. [Crossref] [PubMed]
Cite this article as: Rodriguez HA, Oelschlager BK. Secrets for successful laparoscopic antireflux surgery: mesh hiatoplasty. Ann Laparosc Endosc Surg 2017;2:50.