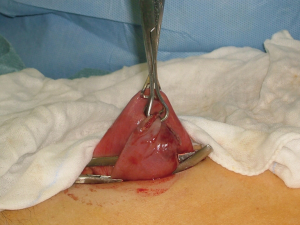
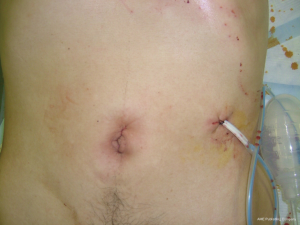
Dual port distal gastrectomy
IntroductionOther Section
- Introduction
- Patient selection and working
- Preoperative preparation
- Procedure
- Role of team members
- Post-operative management
- Tips, tricks and pitfalls
- Acknowledgments
- Footnote
- References
The introduction of laparoscopic surgery has resulted in significant improvements in outcomes for a range of surgical procedures. A number of studies show that laparoscopic surgery is associated with better outcomes such as shorter length of stay, reduced surgical complications, reduced bleeding and pain, compared with open surgery (1,2). This procedure is spreading widely in various fields. A recent challenge in the performance of laparoscopic surgery is to reduce the number of incisions to lessen invasiveness. Single incision laparoscopic surgery (SILS) or reduced port surgery (RPS) is one of this approach. Although the surgical procedure and equipment of laparoscopic surgery has been developed in various fields, laparoscopic gastrectomy (LAG) is still selective because of the complexity of the procedure (3,4). To complete this operation, four or five incisions (ports) or additional small incisions are generally required (5,6). These assistant incisions or ports are needed for visualization of the working field and/or making the counter traction against dissected tissues during LAG.
Multi-channel ports are spreading worldwide to achieve less invasiveness. This port enables insertion of multiple instrument via a single incision. SILS and RPS which use a multi-channel port to reduce the invasiveness of the surgery has been reported (7-9). Previously, we reported the benefits of RPS for early gastric cancer (10). Our concept is “do not use a useless port”. Here we demonstrate the technical aspect of dual port distal gastrectomy.
Patient selection and workingOther Section
- Introduction
- Patient selection and working
- Preoperative preparation
- Procedure
- Role of team members
- Post-operative management
- Tips, tricks and pitfalls
- Acknowledgments
- Footnote
- References
Eighteen patients (5 women, 13 men) were attended in this study between December 2010 and December 2014. Dual port distal gastrectomies were operated on by the same surgeon in one institution. The patient’s mean age and body mass index (BMI) were 67.6 years (range, 50–87) and 22.2 kg/m2 (range, 16.7–28.4), respectively. Seven (38.9%) of the patients were over 75 years old. As shown in Table 1, several patients had co-morbidities, such as diabetes, hypertension, cardiovascular disease, chronic obstructive pulmonary disease, chronic renal failure and past history of cerebral infarction. For evaluation of patients’ activity, the grade of the Eastern Cooperative Oncology Group (ECOG) performance status (11) was used.
Table 1
| Characteristic | n |
|---|---|
| Age (mean ± SD) | 50–87 (67.6±10.8) |
| Male/female | 13/5 |
| Mean BMI (kg/m2) | 22.2±3.26 |
| Performance status | |
| 0 | 13 |
| 1 | 2 |
| 2 | 3 |
| Comorbid diseases | |
| Diabetes mellitus | 2 |
| Hypertension | 3 |
| Cardio vascular | 5 |
| Respiratory | 1 |
| CRF | 1 |
| Cerebral infarction | 1 |
BMI, body mass index; CRF, chronic renal failure.
Seventeen patients were diagnosed with early gastric cancer located in the middle and distal third portion of the stomach by preoperative biopsy specimens and other imaging studies including endoscopic findings and CT scan. Contra-indication cases of endoscopic submucosal dissection (ESD) such as larger size, deeper invasion and pathologically undifferentiated type of tumors were selected for dual port distal gastrectomy. This indication was according to guidelines of the Japanese Gastric Cancer Association (12). A case of duodenal stricture due to chronic duodenal ulcer disease was also selected.
Preoperative preparationOther Section
- Introduction
- Patient selection and working
- Preoperative preparation
- Procedure
- Role of team members
- Post-operative management
- Tips, tricks and pitfalls
- Acknowledgments
- Footnote
- References
Because the tumors could not be visualized laparoscopically, Indian ink was injected in the proximal side of the lesion by preoperative endoscopy. This procedure enables to recognize the location of cancer lesions laparoscopically. As the multichannel port, the SILS Port (Covidien, Tokyo, Japan) was used. To avoid confliction of surgical instrument, 5-mm flexible laparoscope (Olympus, Tokyo, Japan) were prepared in all cases. Patients were allowed to have conventional meals the day before surgery, and drink clear liquids 2 hours before surgery.
ProcedureOther Section
- Introduction
- Patient selection and working
- Preoperative preparation
- Procedure
- Role of team members
- Post-operative management
- Tips, tricks and pitfalls
- Acknowledgments
- Footnote
- References
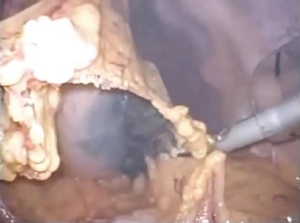
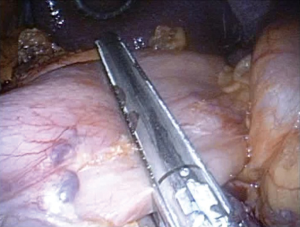
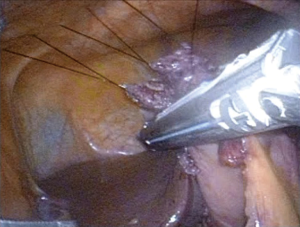
Surgeries were performed by a single surgeon under general anesthesia in the lithotomy position. Pneumoperitoneum pressure was preset at 10 mmHg. After one 2.5-cm transumbilical incision was made in a vertical fashion, the SILS Port was inserted via this vertical incision. Normally a surgeon was positioned between the patient’s legs during laparoscopic procedure (Figure 1). After pneumoperitoneum was created by CO2 input tube to the inlet in the port, the visceral space was inspected. Then, a second port, 12-mm in size, was inserted into the left lower quadrant of abdomen. This port was mainly used for the surgeon’s right hand, and an Endo-GIA (Covidien, Tokyo, Japan) or suture devises was also used by this 12-mm port. To visualize the neighboring organs and bursa space, the gastric wall was lifted by the surgical nylon ligature. A surgical nylon ligature with a straight needle was pricked on the skin in the upper abdomen and inserted into the abdominal cavity without any ports. And this ligature was simply sutured to the anterior wall of the stomach (Figure 2). After making two sutures into the stomach wall, the needles were removed from the abdominal cavity via the abdominal wall. Then, the stomach wall was lifted manually and fixed temporarily to the abdominal wall. This procedure enabled to visualize the relationship between the gastric vessels and neighboring organs (Figure 3). After opening bursa space by the hemostat device such as Harmonic Scalpel (Johnson & Johnson, Tokyo, Japan), the gastric vessels were recognized easily. Then, the distal side of gastroepiploic arcade and right gastric artery were dissected and cut with clipping and hemostat device. The proximal side of gastroepiploic vessels were also dissected and cut along the proximal side of the stain that had been injected preoperatively. The Endo-GIA was used to cut the duodenum. After cutting the duodenum, the left gastric vessels such as the left gastric artery and coronary vein were exposed and cut near the root. The proximal side of stomach was cut along the stain by the Endo-GIA and distal gastrectomy was completed (Figure 4). Then, lymph node dissection around the common hepatic and celiac artery was performed. We performed Roux-en-Y reconstruction in all cases. After making a gastro-jejunostomy by the Endo-GIA, the suture hole was simply sutured and lifted up by the nylon ligature in the same manner as before, and closed by the Endo-GIA (Figure 5). After removing SILS Port, Jejuno-jejunostomy was made manually using the 2.5-cm incision of umbilical port site (Figure 6). A flat closed drain was inserted around the gastro-jejunostomy using the left 12 mm port site (Figure 7).
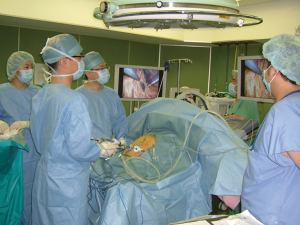
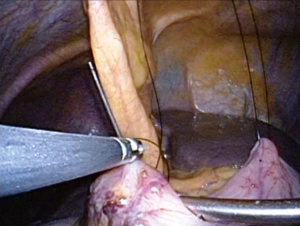
Role of team membersOther Section
- Introduction
- Patient selection and working
- Preoperative preparation
- Procedure
- Role of team members
- Post-operative management
- Tips, tricks and pitfalls
- Acknowledgments
- Footnote
- References
- Hiroyuki Kashiwagi: performed dual port gastrectomies, interpreted the results, drafted and finalized the manuscript.
- Jun Kawachi: critically revised the manuscript.
- Naoko Isogai: trainee for RPS.
- Takako Yamanashi: collection and assembly of data.
- Katsunori Miyake: trainee for RPS.
- Rai Shimoyama: conducted experiments.
- Ryota Fukai: conducted experiments.
- Yasushi Terada: conducted experiments.
- Hidemitsu Ogino: critically revised the manuscript.
Post-operative managementOther Section
- Introduction
- Patient selection and working
- Preoperative preparation
- Procedure
- Role of team members
- Post-operative management
- Tips, tricks and pitfalls
- Acknowledgments
- Footnote
- References
All patients were moved from the high care unit to the general unit the day after surgery and began to take soft meals. Rehabilitation was also started on postoperative day (POD) 1. Drain was normally removed on POD 3. Laboratory data were obtained on PODs on 1 and 3 in all cases. Table 2 shows the outcomes of the dual port surgery. As we had reported previously, the surgical outcomes were acceptable, compared to the conventional 4 or 5 ports method (9). No major complications have been occurred in all cases. The safety surgical margins were also confirmed by pathological study. Because every cancer patient had no lymph node metastasis, an additional chemotherapy or surgery was not required in all cases, according to guidelines of the Japanese Gastric Cancer Association (12).
Table 2
| Variables | Outcomes |
|---|---|
| Operation time (min) | 273.4±36.1 |
| Intra-op. bleeding (mL) | 59.2±86.1 |
| Dissected lymph nodes (No.) | 16.1±2.8 |
| Hospital stay after surgery (days) | 8.3±1.6 |
Tips, tricks and pitfallsOther Section
- Introduction
- Patient selection and working
- Preoperative preparation
- Procedure
- Role of team members
- Post-operative management
- Tips, tricks and pitfalls
- Acknowledgments
- Footnote
- References
Our concept is “Do not use an unnecessary port”. Normally the role of assistant ports is to make counter traction and a working field in the visceral space. To replace this role, a surgical nylon ligature with a straight needle is crucial. This nylon ligature enables to maintaining the visual field without any additional ports or surgical instruments. Because these nylon sutures or related small injury on gastric wall are resected when gastrectomy, the sutures could make repeatedly during operation. If counter traction or the visual field is not sufficient, other sutures without any additional ports could be made. We also use this technique to close the suture hole in gastrojejunostomy. After simple sutures by nylon ligatures, this suture hole was easily closed by the Endo GIA.
The SILS technique has limitation of motion and reduced ability of triangular dissection by surgical instruments. Because a camera and two surgical instruments were introduced into the visceral space through a small umbilical incision, collision and conflict of these instruments are inevitable. Consequently, an inherent technical challenge that arises from the SILS technique is that of a compromised view and locomotive field (13). A multichannel port with small additional port may overcome these problems (10,14). Our method uses the SILS Port and a 12-mm port. This should prevent interference between camera maneuvers and other movement of surgical instruments, and maintain an ergonomic position for the surgeon and assistant.
The 5-mm flexible scope is also crucial. The use of multiple instruments with a multichannel port sometimes results in “sword fighting” between the scope and the other instruments. A 5-mm flexible scope can avoid this problem because the articulating tip can bend in all directions up to 100 degrees. Personally, we consider that 5-mm scope is better than a 10-mm scope to avoid the same problem although the 10-mm scope generally provides a better imaging performance. In combination with a multichannel port, an additional 12-mm port, a 5-mm flexible scope, and the surgical nylon ligature with straight needle enable the achievement of dual port surgery, even for LAG.
A needle device such as Endo-relief forceps (Hope Denshi Co., Chiba, Japan) is a future option (15). Endo-relief was used for two cases in this series. Its usage is not necessary but provides easy access, especially for persons unfamiliar with SILS or RPS.
AcknowledgmentsOther Section
- Introduction
- Patient selection and working
- Preoperative preparation
- Procedure
- Role of team members
- Post-operative management
- Tips, tricks and pitfalls
- Acknowledgments
- Footnote
- References
Funding: None.
FootnoteOther Section
- Introduction
- Patient selection and working
- Preoperative preparation
- Procedure
- Role of team members
- Post-operative management
- Tips, tricks and pitfalls
- Acknowledgments
- Footnote
- References
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chikara Kunisaki) for the series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.03.11). The series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval was waived. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- Patient selection and working
- Preoperative preparation
- Procedure
- Role of team members
- Post-operative management
- Tips, tricks and pitfalls
- Acknowledgments
- Footnote
- References
- Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005;6:477-84. [Crossref] [PubMed]
- Buunen M, Veldkamp R, Hop WC, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009;10:44-52. [Crossref] [PubMed]
- Kitano S, Yasuda K, Shiraishi N. Laparoscopic surgical resection for early gastric cancer. Eur J Gastroenterol Hepatol 2006;18:855-61. [Crossref] [PubMed]
- Zhang X, Tanigawa N. Learning curve of laparoscopic surgery for gastric cancer, a laparoscopic distal gastrectomy-based analysis. Surg Endosc 2009;23:1259-64. [Crossref] [PubMed]
- Coratti A, Annecchiarico M, Di Marino M, et al. Robot-assisted gastrectomy for gastric cancer: current status and technical considerations. World J Surg 2013;37:2771-81. [Crossref] [PubMed]
- Kim HH, Ahn SH. The current status and future perspectives of laparoscopic surgery for gastric cancer. J Korean Surg Soc 2011;81:151-62. [Crossref] [PubMed]
- Kanehira E, Siozawa K, Kamei A, et al. Development of a novel multichannel port (x-Gate((R))) for reduced port surgery and its initial clinical results. Minim Invasive Ther Allied Technol 2012;21:26-30. [Crossref] [PubMed]
- Kashiwagi H, Kumagai K, Nozue M. Single incision laparoscopic surgery for a life-threatening, cyst of liver. Tokai J Exp Clin Med 2011;36:13-6. [PubMed]
- Kashiwagi H, Kumagai K, Nozue M, et al. Morgagni hernia treated by reduced port surgery. Int J Surg Case Rep 2014;5:1222-4. [Crossref] [PubMed]
- Kashiwagi H, Kumagai K, Monma E, et al. Dual-port distal gastrectomy for the early gastric cancer. Surg Endosc 2015;29:1321-6. [Crossref] [PubMed]
- No JH, Kim SW, Lim CH, et al. Long-term outcome of palliative therapy for gastric outlet obstruction caused by unresectable gastric cancer in patients with good performance status: endoscopic stenting versus surgery. Gastrointest Endosc 2013;78:55-62. [Crossref] [PubMed]
- Tanabe S, Ishido K, Higuchi K, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer 2014;17:130-6. [Crossref] [PubMed]
- Froghi F, Sodergren MH, Darzi A, et al. Single-incision Laparoscopic Surgery (SILS) in general surgery: a review of current practice. Surg Laparosc Endosc Percutan Tech 2010;20:191-204. [Crossref] [PubMed]
- Hirano Y, Hattori M, Douden K, et al. Single-incision plus one port laparoscopic anterior resection for rectal cancer as a reduced port surgery. Scand J Surg 2012;101:283-6. [Crossref] [PubMed]
- Sakamoto K, Okazawa Y, Takahashi R, et al. Laparoscopic intersphincteric resection using needlescopic instruments. J Minim Access Surg 2014;10:221-4. [Crossref] [PubMed]
Cite this article as: Kashiwagi H, Kawachi J, Isogai N, Yamanashi T, Miyake K, Shimoyama R, Fukai R, Terada Y, Ogino H. Dual port distal gastrectomy. Ann Laparosc Endosc Surg 2017;2:59.