
Technical aspects and surgical complications of laparoscopic liver resection
Introduction
Liver resection (LR) is one of the curative strategies for various liver tumors, including primary malignant tumors, metastatic liver tumors, and some benign tumors. Laparoscopic LR (Lap LR) was first reported in 1991 (1). The indications for Lap LR are expanding, and the feasibility of performing major hepatectomy using a laparoscopic procedure has been proven at expert institutions (2,3). The main clinical benefits of Lap LR compared with open surgery are that it is less invasive, causes less pain, and results in a fast recovery after surgery (4). On the other hand, the demerits of Lap LR include its technical difficulty, the safety of such surgery, the longer operation time, and increased operating theater costs. One of the aims of this review is to discuss the technical aspects of Lap LR, including the difficulty score (DS) and intraoperative blood flow control.
Although Lap LR results in fewer postoperative complications than open surgery, biliary duct complications require careful attention. As the surgeon cannot hold the liver parenchyma with their left hand to control hemostasis during Lap LR procedures, parenchymal ablation is necessary to achieve hemostasis. Various ablation devices have been developed for use during surgery. They can be used to seal blood vessels (by denaturing the chemical structure of collagen fibers) during surgery, and bile ducts can be sealed via a similar mechanism. However, unexpected biliary complications can occur because the ablated area is hard to estimate during surgery. We encountered a case in which biliary stricture occurred as a late surgical complication of Lap LR, and endoscopic intervention was required in this case. The second aim of this review is to caution against the use of ablating devices adjacent to the major Glissonian pedicle.
DS
The difficulty of LR is determined by the type of operation and the degree of central obesity (5). A complexity score and a classification of LR, which were based on expertise from all over the world, were proposed for open LR (6). Twelve procedures were rated and divided into three grades, low, medium, and high complexity. The “A Body Shape Index” (ABSI), which is based on waist circumference (WC), adjusted for height and weight, represents body habitus (7). Unlike body mass index, the ABSI distinguishes between peripheral and central fat (which can affect clinical outcomes after Lap LR) (5). Although the ABSI was shown to be a substantial risk factor for premature mortality in the general population (7), the ABSI and the difficulty of LR are also associated with the conversion risk (5). Interestingly, the ABSI was only correlated with the operation time in the high difficulty group (5). The DS for Lap LR is summarized in Table 1 (8). This score takes into account the location of the resection site, the surgical procedure, tumor size, the of the resection site distance from major blood vessels, and liver function (8). The score is calculated based on mathematical equations. It has been validated in a retrospective study involving 78 patients who underwent Lap LR, and the DS was found to be correlated with the operation time and intraoperative blood loss (the mean values were 256 min and 168 mL, respectively) (9). Another validation study also obtained similar results (10), but it did not detect a relationship between the DS and the operation time. Although the DS does not take body habitus into account, it can be used for both technical evaluations at single institutions and comparisons of surgical outcomes among multiple institutions.
Table 1
| Variables | Score |
|---|---|
| Tumor location | |
| S2 | 2 |
| S3 | 1 |
| S4 | 3 |
| S5 | 3 |
| S6 | 2 |
| S7 | 5 |
| S8 | 5 |
| Total | A |
| Extent of liver resection | |
| Hr0 | 0 |
| Hr-LLR | 2 |
| Hr-S | 3 |
| Hr-1, 2 | 4 |
| Total | B |
| Tumor size | |
| <3 cm | 0 |
| >3 cm | 1 |
| Total | C |
| Proximity to major vessels | |
| No | 0 |
| Yes | 1 |
| Total | C |
| Liver function | |
| Child-Pugh A | 0 |
| Child-Pugh B | 1 |
| Total | C |
Difficulty score (DS) = A + B + C (range: 1–12). DS: 1–3, low grade; 4–6, intermediate grade; and 7–10, high grade. S, segment; Hr, hepatic resection; LLR, left lateral sectionectomy.
Evaluation of the DS in our case series
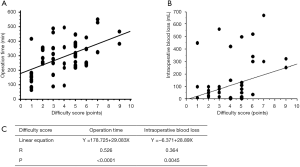
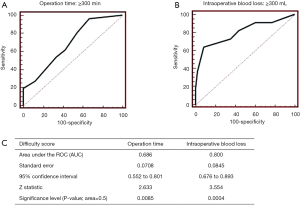
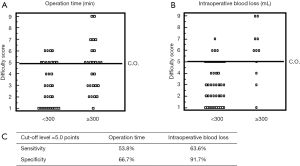
The cases of 88 consecutive patients who underwent pure Lap LR at our institution between July 2010 and December 2016 were retrospectively evaluated. The patients’ clinical data are shown in Table 2. The mean operation time was 257.8 min, and the median amount of intraoperative blood loss was 20 mL. The mean DS was 3.7. The results of a linear regression analysis of the relationships between the DS and the operation time or intraoperative blood loss are shown in Figure 1. The DS was found to be correlated with the operation time (R=0.526, P<0.0001) and the amount of intraoperative blood loss (R=0.364, P=0.0045). The regression coefficients for these relationships indicate that the operation time and amount of intraoperative blood loss increase by 30 min and 30 mL, respectively, for every DS point. Furthermore, the utility of the DS for predicting an operation time of 300 min or intraoperative bleeding of 300 mL was assessed based on receiver operating curve (ROC) analysis (Figures 2A,B). The area under the ROC curve for these parameters was 0.686 and 0.800, respectively (Figure 2C). The optimal DS cut-off values for predicting these outcomes were evaluated using a dot-blot diagram (Figure 3) and was found to be 5 DS points in both cases. Using this cut-off value, the DS exhibited sensitivity and specificity values of 53.8% and 66.7%, respectively, for predicting an operation time of ≥300 min. On the other hand, it displayed sensitivity and specificity values of 63.6% and 91.7%, respectively, for predicting intraoperative blood loss of ≥300 mL. Therefore, the DS could be useful for predicting the risk of a long operation and increased intraoperative blood loss. Patients with DS of ≥5 points could be at high risk of requiring an operation lasting at least 300 min and/or involving at least 300 mL of intraoperative blood loss. These roles may be applied in our institute solely, but self-evaluation should be considered at each institution. It might be possible to compare the clinical outcomes of different institutions by matching patients using the DS.
Table 2
| Variables | Median or mean | IQR or SD | Kolmogorov-Smirnov test |
|---|---|---|---|
| Age (years) | 67.0 | 61–72 | 0.017 |
| Sex (male: female) | 40:48 | ||
| Albumin (g/dL) | 3.9 | 3.3–4.1 | 0.001 |
| Bilirubin (mg/dL) | 0.7 | 0.5–1.0 | 0.003 |
| PT (%) | 89.2 | 12.5 | 0.897 |
| ICG-R15 (%) | 10.0 | 6.5–16.45 | 0.002 |
| Platelets (/104 mm2) | 15.9 | 10.6–20.0 | 0.001 |
| Tumor size (cm) | 2.7 | 1.7 | 0.115 |
| Tumor numbers | 1 | 0–1 | 0.001 |
| Hr (0:1:2) | 79:7:2 | ||
| Op time (min) | 257.8 | 117.8 | 0.813 |
| Intraoperative blood loss (mL) | 20 | 0–112 | 0.001 |
| Difficulty score (points) | 3.7 | 2.1 | 0.184 |
IQR, interquartile range; SD, standard deviation; PT, prothrombin time; ICG-R15, indocyanine green retention at 15 min; Hr 0, partial resection; Hr 1, mono-segmentectomy; Hr 2, bi-segmentectomy.
In fact, a comparison of our experience and the cases described in the study by Im et al. is shown in Table 3. While higher DS were recorded in Im’s study, the mean operation time was shorter, and the mean amount of intraoperative blood loss was greater than in our cases. It would not be appropriate to perform simple comparisons between the clinical outcomes of the two studies as they exhibited different DS, which might have been due to variations in the patients’ backgrounds. The abovementioned findings could imply that different surgical policies are in operation at the two institutions; i.e., that our surgical team regard intraoperative bleeding as the most important factor but Im’s team regard the operation time as the most important factor. Therefore, the DS could also aid comparisons of the clinical outcomes of Lap LR among different circumstances.
Table 3
| Variables | Median or mean | IQR or SD |
|---|---|---|
| Our experience | ||
| DS | 3.7 | 2.1 |
| Op time | 257.8 | 117.8 |
| Intraoperative blood loss | 20 | 0–112 |
| Im et al. | ||
| DS | 4.7 | 3–6* |
| Op time | 160 | 152–200* |
| Intraoperative blood loss | 300 | 64–300* |
*, range. DS, difficulty score; Op, operation; IQR, interquartile range; SD, standard deviation.
Blood flow control (Table 4)
Table 4
| Years | Authors | Strategy |
|---|---|---|
| 1908 | Pringle | Pringle maneuver |
| 1987 | Makuuchi | Hemihepatic vascular occlusion |
| 1986 | Shimamura | Selective vascular control of segmental branches |
| 2000 | Cherqui | Intracorporeal hepatic inflow control |
| 2008 | Belli | Surround the hepatoduodenal ligament using the Endo Retract Maxi |
| 2009 | Cho | Extracorporeal hepatic inflow control using a short tube |
| 2012 | Rotellar | Extracorporeal hepatic inflow control using a long tube |
| 2015 | Mizuguchi | Extracorporeal hepatic inflow control using a long tube (patents submitted in Japan and the US in 2010 and 2011, respectively) |
Various blood flow control methods have been invented, such as inflow and outflow control methods, for both open and Lap LR. In this review, we focus on inflow control methods. In open surgery, Pringle described the total clamping of the hepatoduodenal ligament in a traumatic case in 1908 (11). Makuuchi et al. reported a hemihepatic vascular occlusion technique for reducing the risk of organ congestion and hepatic ischemia (12). Furthermore, Shimamura et al. achieved selective vascular control of the segmental branches using a balloon catheter during LR (13). In Lap LR, various blood flow control techniques have been reported.
An intracorporeal method was described by Cherqui et al. in 2000 (14). However, difficult cases in which it was not possible to obtain good blood flow control were sometimes encountered, as it can be hard to squeeze the tourniquet sufficiently using laparoscopic devices. Extracorporeal hepatic inflow control methods were subsequently reported. Cho et al. reported an extracorporeal method involving the use of a short tube in 2009 (15). Belli et al. (16) stated that they described this technique earlier than Cho et al.; however, Belli et al.’s method advantage involved surrounding the hepatoduodenal ligament with the Endo Retract Maxi. We also came up with a similar extracorporeal inflow control method at a similar time. Our idea was based on the use of a long tube, which was placed at the bottom of the surgical field. This made it possible to secure the surgical working space. We prepared to protect our idea and submitted patents regarding our extracorporeal method on Oct 5, 2010, in Japan and on Oct 5, 2011, in the US. Eventually, Rotellar et al. reported a similar idea; i.e., they used a long tube during the extracorporeal Pringle maneuver, in 2012 (17). Our paper was delayed and was not published until 2015 (18) due to the long process involved in getting patent rights in Japan and the US. Our clamping device is commercially available as the vClumpTM (Kono Seisakusho: Crownjun, Tokyo, Japan). We could not name it the vClampTM due to commercial rights issues in Japan.
Biliary stricture after Lap LR
Lap LR is less invasive than open surgery. Surgical energy devices, such as radiofrequency (RF)-based energy devices and bipolar ultrasound coagulators, play an important role in obtaining good hemostasis and as well as in Lap LR itself. Table 5 shows representative liver parenchymal dissection methods (19). In the classic method, which was in use at the end of the last century, the liver parenchyma is dissected manually and mechanically (the upper section in Table 5), and most vessels and bile ducts are ligated. On the other hand, sealing devices based on ultrasound oscillation, monopolar ablation, bipolar ablation, or RF ablation, are employed in modern methods (the lower section in Table 5). Among these methods for dissecting the liver parenchyma, RF ablation has been found to result in high bile duct injury-associated morbidity rates. Janssen et al. also reported that 4 out of 122 patients suffered biliary stricture and required multiple endoscopic procedures (20). The clinical symptoms of the bile duct stricture in these cases occurred late (from 1 to 4 months after surgery). A randomized controlled trial of the RF and clamp crushing methods showed that both techniques produced similar clinical outcomes, but the bile duct injury-related morbidity rate was significantly higher in the RF group than in the clamp crushing group. In the latter study, one out of 24 patients in the RF group suffered biliary stenosis (21). Clinicians should be aware that bile duct stricture can occur as a severe late complication after Lap LR.
Table 5
| Techniques and surgical devices | Year | First author |
|---|---|---|
| Finger fracture technique (digitoclasy) | 1960 | Lin |
| Kelly (clamp crushing) | 1974 | Lin |
| Ultrasonic dissector (CUSA, Tyco Healthcare, Mansfield, MA, USA) | 1992 | Hodgson |
| Hydro-Jet (Erbe, Tubingen, Germany) | 1993 | Baer |
| Harmonic scalpel (Ultracision, Ethicon Endo-surgery, Cincinnati, OH, USA) | 2000 | Gertsch |
| Dissecting sealer (Tissuelink, Dover, NH, USA) | 2005 | Arita |
| Radiofrequency generator (RFALR)* | 2005 | Haghighi |
*, RFALR can be performed with the Habib and LigaSure devices (Medtronic, Minneapolis, MN, USA). RFALR, radiofrequency-assisted liver resection.
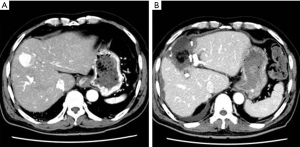
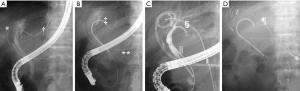
A representative case of post Lap LR biliary stricture is shown in Figure 4. A 70-year-old male had hepatocellular carcinoma (diameter: 3 cm) in segment 4a of the liver. The affected segment and the tumor were removed via a pure laparoscopic approach without any complications (Figure 4B). However, bile leakage was seen from the stump of B4 because the internal pressure in the affected region had increased due to bile duct stricture in B2 + B3. First, external drains were placed in the bile cavity (Figure 5A). Then, endoscopic retrograde biliary drainage of B2 was performed (Figure 5B). Subsequently, bile duct stricture developed in the B3 branch. Percutaneous drainage of B3 was carried out, and B3 was recanalized through the B4 stump using the rendezvous technique (Figure 5C), which was similar to the technique reported by Deviere (22). Eventually, internal drainage of bile ducts B2 and B3 was performed (Figure 5D). The successful treatment of biliary morbidities requires organized planning and care from a multidisciplinary team. Although Lap LR is less invasive, biliary complications can arise as significant morbidities. It is better to consult a tertiary center before considering the surgical approach in cases in which biliary stricture occurs after Lap LR.
Conclusions
We have reviewed the utility of the DS for evaluating the technical difficulty of Lap LR using our case series. The development of inflow blood control methods was also reviewed. Furthermore, we have highlighted that bile duct stricture can arise as a significant complication after Lap LR and is associated with RF-based heat injuries. Consultation with a tertiary center and multidisciplinary planning should be considered in cases involving post-LAP LR biliary stricture.
Acknowledgments
We thank Dr. Hirata for his service in our department during the last quarter century.
Funding: This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (No. 26461921) to T Mizuguchi.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.02.25). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reich H, McGlynn F, DeCaprio J, et al. Laparoscopic excision of benign liver lesions. Obstet Gynecol 1991;78:956-8. [PubMed]
- van der Poel MJ, Besselink MG, Cipriani F, et al. Outcome and Learning Curve in 159 Consecutive Patients Undergoing Total Laparoscopic Hemihepatectomy. JAMA Surg 2016;151:923-8. [Crossref] [PubMed]
- Cheek SM, Geller DA. The Learning Curve in Laparoscopic Major Hepatectomy: What Is the Magic Number? JAMA Surg 2016;151:929. [Crossref] [PubMed]
- Mizuguchi T, Kawamoto M, Meguro M, et al. Laparoscopic hepatectomy: a systematic review, meta-analysis, and power analysis. Surg Today 2011;41:39-47. [Crossref] [PubMed]
- Ratti F, D'Alessandro V, Cipriani F, et al. Influence of body habitus on feasibility and outcome of laparoscopic liver resections: a prospective study. J Hepatobiliary Pancreat Sci 2016;23:373-81. [Crossref] [PubMed]
- Lee MK 4th, Gao F, Strasberg SM. Perceived complexity of various liver resections: results of a survey of experts with development of a complexity score and classification. J Am Coll Surg 2015;220:64-9. [Crossref] [PubMed]
- Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One 2012;7:e39504 [Crossref] [PubMed]
- Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745-53. [Crossref] [PubMed]
- Uchida H, Iwashita Y, Saga K, et al. Clinical Utility of the Difficulty Scoring System for Predicting Surgical Time of Laparoscopic Liver Resection. J Laparoendosc Adv Surg Tech A 2016;26:702-6. [Crossref] [PubMed]
- Im C, Cho JY, Han HS, et al. Validation of difficulty scoring system for laparoscopic liver resection in patients who underwent laparoscopic left lateral sectionectomy. Surg Endosc 2017;31:430-6. [Crossref] [PubMed]
- Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg 1908;48:541-9. [Crossref] [PubMed]
- Makuuchi M, Mori T, Gunvén P, et al. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet 1987;164:155-8. [PubMed]
- Shimamura Y, Gunvén P, Takenaka Y, et al. Selective portal branch occlusion by balloon catheter during liver resection. Surgery 1986;100:938-41. [PubMed]
- Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 2000;232:753-62. [Crossref] [PubMed]
- Cho A, Yamamoto H, Nagata M, et al. Safe and feasible inflow occlusion in laparoscopic liver resection. Surg Endosc 2009;23:906-8. [Crossref] [PubMed]
- Belli G, Fantini C, D'Agostino A, et al. Re: Safe and feasible inflow occlusion in laparoscopic liver resection. Surg Endosc 2009;23:1686. [Crossref] [PubMed]
- Rotellar F, Pardo F, Bueno A, et al. Extracorporeal tourniquet method for intermittent hepatic pedicle clamping during laparoscopic liver surgery: an easy, cheap, and effective technique. Langenbecks Arch Surg 2012;397:481-5. [Crossref] [PubMed]
- Mizuguchi T, Kawamoto M, Nakamura Y, et al. New technique of extracorporeal hepatic inflow control for pure laparoscopic liver resection. Surg Laparosc Endosc Percutan Tech 2015;25:e16-20. [Crossref] [PubMed]
- Galizia G, Castellano P, Pinto M, et al. Radiofrequency-assisted liver resection with a comb-shaped bipolar device versus clamp crushing: a clinical study. Surg Innov 2012;19:407-14. [Crossref] [PubMed]
- Jansen MC, van Duijnhoven FH, van Hillegersberg R, et al. Adverse effects of radiofrequency ablation of liver tumours in the Netherlands. Br J Surg 2005;92:1248-54. [Crossref] [PubMed]
- Lupo L, Gallerani A, Panzera P, et al. Randomized clinical trial of radiofrequency-assisted versus clamp-crushing liver resection. Br J Surg 2007;94:287-91. [Crossref] [PubMed]
- Devière J. Benign Biliary Strictures and Leaks. Gastrointest Endosc Clin N Am 2015;25:713-23. [Crossref] [PubMed]
Cite this article as: Nagayama M, Mizuguchi T, Yamaguchi H, Imamura M, Kutomi G, Okita K, Nishidate T, Takemasa I, Kimura Y. Technical aspects and surgical complications of laparoscopic liver resection. Ann Laparosc Endosc Surg 2017;2:61.


