减孔腹腔镜近端胃癌切除术的技术进展
引言
胃癌是最常见的恶性肿瘤之一,是全球癌症死亡的第二大原因[1]。自从Kitano等人首次报道成功实施腹腔镜辅助远端胃切除术(laparoscopy-assisted distal gastrectomy,LADG)治疗早期胃癌后,许多临床试验也已经证实了这一术式的益处[2-4]。腹腔镜胃切除术的常规技术通常需要5枚或6枚戳卡孔[5-7]。
RPS作为一种新颖的手术理念,包括单孔腹腔镜手术(single incision laparoscopic surgery,SILS)和针式腹腔镜手术(needlescopic surgery,NS),使用的戳卡孔比传统手术更少或更小,相比传统腹腔镜手术降低了手术侵入性,并增加美容效果[8,9],在普通外科、妇科和泌尿外科等领域均报道了各自减孔手术流程[10,11]。一些报道对胃癌的减孔手术中涉及手术技巧和可操作性上进行了描述。然而,由于缺少“三角原则”,以及器械碰撞所带来的技术困难,腹腔镜胃切除术很少实施减孔手术,特别是对于近端三分之一的胃癌[12-14]。
相比全胃切除术,PG是早期近端三分之一胃癌的功能保留手术的更好选择,可以最大限度地减少胃切除术后的后遗症并提高胃切除术后的生活质量[15-18]。尽管已有众多关于近端胃切除术后的各种重建方式(如食管胃造口术、双道、空肠袋置入术和空肠置入术)的报道,但尚未就最佳重建方式达成共识。在使用这些重建方法进行近端胃切除术后,最麻烦的问题是食管胃吻合术引起的反流症状和手术后内镜监测困难。为了解决这些问题,Kamikawa等人开发了一种包含一个回流阀的食管胃吻合术[19]。我们可以应用这种方法对近端胃癌进行减孔腹腔镜手术,以最大限度地减少手术侵入性,改善美容效果,并预防术后反流症状。
本章介绍了我们使用椭圆形端口装置和针式腹腔镜进行减孔腹腔镜近端胃癌切除术(RPPG)涉及的技术。
适应证与禁忌证
减孔腹腔镜近端胃癌切除术适应于早期近端三分之一胃癌。对于cT1cN0肿瘤,日本胃癌治疗指南推荐近端癌进行近端胃切除术加D1+淋巴结清扫术,以保留一半以上的远端胃[20]。还包括食管胃结合部腺癌(AEG),即内镜下分类为Siewert II型肿瘤,无论肿瘤浸润深度如何。但病态肥胖(BMI<35 kg/m2)、肿瘤侵犯邻近器官以及大块肿瘤(<8 cm)的患者予以排除。
技术
体位
患者在全身麻醉下置于头高脚底位(反Trendelenburg位)。主刀医生站在患者的右侧,扶镜手站在患者双腿之间,助手位于患者左侧。
戳卡孔布局、接入设备和针式腹腔镜设备
我们的减孔腹腔镜近端胃癌切除术中采用了两种戳卡布局方式。第一种是中尺寸的单孔腹腔镜装置[21,22](E-Z通道椭圆形0707D LAP PROTECTORTM Hakko有限公司,日本东京;图1)使用30 mm镜头戳卡经脐置入腹腔和三枚2.7 mm戳卡孔(MiniportTM;Medtronic,爱尔兰都柏林)分别位于右上腹和左上腹。MiniportTM经皮穿刺置入。这种方法称为针式腹腔镜手术(带3枚穿刺孔的RPPG),尽管戳卡数与传统腹腔镜近端胃切除手术相同,但戳卡孔的尺寸缩小(图2)。
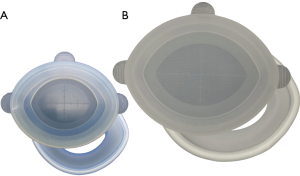
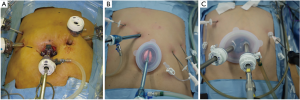
另一种方法为大尺寸单孔腹腔镜装置(椭圆形E-Z accessTM搭配椭圆形LAPPROTECTOR™ 1010HD,Hakko有限公司;图1),使用30 mm镜头戳卡经脐置入腹腔,3枚2.7 mm戳卡(MiniportTM;Medtronic,爱尔兰都柏林)分别置于右上腹和左上腹(RPPG+两次穿刺,图2C)。这种情况下,助手戳卡的数量从2枚减少到1枚。外科医生的右手戳卡从右上象限改为E-Z通道椭圆形1010HD型,减少一处右上象限12 mm皮肤切口。
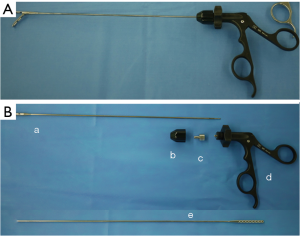
我们的减孔腹腔镜近端胃切除术通过微型戳卡置入针式腹腔镜装置(Endorelif装置, Hope Denshi有限公司,日本千叶),该装置具有直径2.4 mm的轴杆和直径5 mm的钳口™ (图3)。第一种戳卡布局方式中使用了3枚Endorelif设备,后一种布局使用2枚。
尽管戳卡分布不同,但两种情况下的手术操作步骤相同。
肝脏悬吊
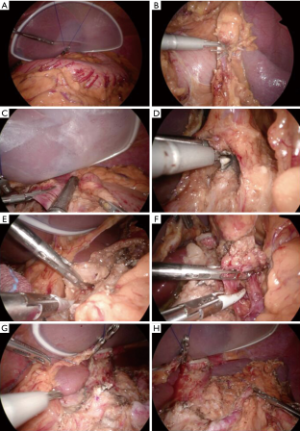
在近端胃手术期间,无损伤的肝脏悬吊对于保持最佳手术视野至关重要。将一个大尺寸的硅胶盘(Hakko公司,日本东京)放入腹腔并置于肝脏外侧段下方。用带有2-0线的直针刺穿上腹部左侧的皮肤并导入腹腔。针穿过上腹部右侧的皮肤。将一个游离钳口(free jaw,FJ)夹(图4,日本Sabae CHARMANT)放入腹腔。用FJ夹将线固定在胃膈韧带上(图5A)。将线的体外双侧末端拔出,用线和硅盘将肝脏悬吊。线尾用夹子固定。
手术步骤
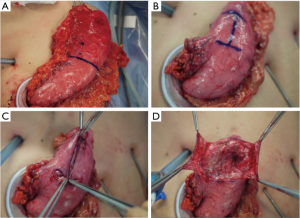
减孔腹腔镜近端胃切除术加D1+淋巴结切除术遵循2010版日本胃癌治疗指南(第3版)[20]。使用SonicBeat(Olympus医疗系统公司,日本东京)和LigaSure Maryland(Medtronic公司,爱尔兰都柏林)进行淋巴结清扫。自大网膜距离胃网膜弓3 cm处开始行大网膜部分切除术,包括第4sb组淋巴结。对胃网膜左血管和胃短血管进行结扎和离断,以清扫第4sb和第4sa组淋巴结(图5B)。继续分离大网膜以游离远端胃,但需保留右侧胃网膜血管以保证远端胃的血供。用腹腔镜线性吻合器在体内切断食管下段(图5C)。在内侧入路之前,分离胃和胰腺的间隙,解剖第9组淋巴结头侧[23]。然后清扫胰腺上区(第7组淋巴结)。找到冠状静脉和胃左动脉后予以结扎和切断(图5D)。沿肝总动脉(图5E)和脾动脉近端(图5F)解剖第8a组和第11p组淋巴结。淋巴结清扫完成后,将E-Z AccessTM椭圆形装置从LAPPROTECTORTM上拆除,以取出胃底(图5G, 图5H, 图6A)。在直接可视化下横断胃。移除离体的近端胃标本。
近端胃切除后,通过体内食管胃吻合术(铰链式双皮瓣法,Kamikawa手术)恢复肠道连续性[19,24]。由于这项手术最初是针对开腹近端胃切除手术的,我们对腹腔镜手术流程行了改进。
在残胃上做用龙胆紫染料标记(横向的字母“H”,大小为3.5 cm×2.5 cm)(图6B)。通过解剖距离残胃前壁顶部和右侧约2 cm处的黏膜下和肌肉层来制作吻合皮瓣(图6C, 图6D)。然后将残胃置回腹腔。在重新安装装置并建立气腹后,进行体内手缝式吻合重建。
残胃上分离出的方形区域顶部和食管后壁(距离残端约5 cm)用3根3-0可吸收线间断缝合(图7A)。在黏膜下层距方形区域底部0.5 cm处切开胃用于吻合(图7B),切口长度与食管直径相同。切开食管残端进行吻合。
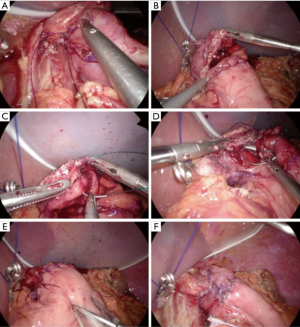
食管后壁与胃吻合时,食管全层和胃黏膜下层用3-0可吸收倒钩缝线缝合(图7C)。缝合后壁后,前壁采用两层吻合(图7D, 图7E)。食管黏膜和胃黏膜黏膜下层采用3-0可吸收带刺线连续缝合。食管肌和胃浆肌层缝合采用7根线间断缝合(图7E)。用双侧吻合皮瓣包裹吻合口(图7F)。将每个皮瓣的全层缝合,并在中心用3-0可吸收倒钩线固定。然后用3-0可吸收倒钩缝线缝合皮瓣底部和胃的浆肌层。然后用4根向上的缝线闭合每个皮瓣。最后,将每个皮瓣分别缝合到食管前壁。重建工作已经完成。在吻合口末端实现Y形皮瓣包裹。食管腔和胃腔之间的压差在吻合口处形成一个快门机制,防止食管反流。
取下右上象限的套管针后,将19或15 Fr硅胶引流管置于左侧膈下间隙,并连接至吸液器(图8A)。
术后腹部切口及功能评估
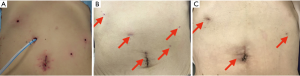
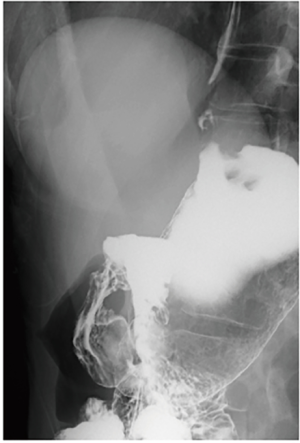
脐切口几乎不明显,仅在手术后一个月微型穿刺孔™处观察到色素沉着,三个月后即消失(图8B, 图8C)。图9显示腹腔镜近端胃切除术术后两个月患者的上消化道造影。即使在15°的头低位,也没有观察到钡剂食管返流。
作者的提示、技巧和建议
NS设备是腹腔镜手术的重要组成部分,并已发展成为取代传统腹腔镜手术的方法之一[25,26]。NS的公认优势包括可忽略的疤痕,潜在的疼痛减轻,发生戳卡疝的风险减少,以及伤口并发症发生率较低。因此,在腹腔镜近端胃切除术中使用2 mm或3 mm的针式戳卡作为附加孔的针孔钳对淋巴结清扫和消化道重建非常有帮助。特别值得一提的是,具有2.4 mm直径轴杆和5 mm直径钳口的EndoRelief装置值得引入腹腔镜手术。通常小直径钳子容易带来如小肠损伤等器官副损伤的问题,但EndoRelief却极少发生,该器械的钳口直径为5 mm,与普通的5 mm钳子使用方法完全相同。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chikara Kunisaki) for the series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” was commissioned by the editorial office without any funding or sponsorship. Dr. Shibao has received patent royalty from Hakko Co. Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 2005;19:168-73. [Crossref] [PubMed]
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20. [Crossref] [PubMed]
- Noshiro H, Nagai E, Shimizu S, et al. Laparoscopically assisted distal gastrectomy with standard radical lymph node dissection for gastric cancer. Surg Endosc 2005;19:1592-6. [Crossref] [PubMed]
- Uyama I, Sakurai Y, Komori Y, et al. Laparoscopy-assisted uncut Roux-en-Y operation after distal gastrectomy for gastric cancer. Gastric Cancer 2005;8:253-7. [Crossref] [PubMed]
- Kanaya S, Kawamura Y, Kawada H, et al. The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. Gastric Cancer 2011;14:365-71. [Crossref] [PubMed]
- Gill IS, Advincula AP, Aron M, et al. Consensus statement of the consortium for laparoendoscopic single-site surgery. Surg Endosc 2010;24:762-8. [Crossref] [PubMed]
- Markar SR, Karthikesalingam A, Thrumurthy S, et al. Single-incision laparoscopic surgery (SILS) vs. conventional multiport cholecystectomy: systematic review and meta-analysis. Surg Endosc 2012;26:1205-13. [Crossref] [PubMed]
- Pfluke JM, Parker M, Stauffer JA, et al. Laparoscopic surgery performed through a single incision: a systematic review of the current literature. J Am Coll Surg 2011;212:113-8. [Crossref] [PubMed]
- Romanelli JR, Earle DB. Single-port laparoscopic surgery: an overview. Surg Endosc 2009;23:1419-27. [Crossref] [PubMed]
- Kunisaki C, Makino H, Yamaguchi N, et al. Surgical advantages of reduced-port laparoscopic gastrectomy in gastric cancer. Surg Endosc 2016;30:5520-8. [Crossref] [PubMed]
- Inaki N. Reduced port laparoscopic gastrectomy: a review, techniques, and perspective. Asian J Endosc Surg 2015;8:1-10. [Crossref] [PubMed]
- Lee CM, Park DW, Jung DH, et al. Single-Port Laparoscopic Proximal Gastrectomy with Double Tract Reconstruction for Early Gastric Cancer: Report of a Case. J Gastric Cancer 2016;16:200-6. [Crossref] [PubMed]
- Nakamura M, Yamaue H. Reconstruction after proximal gastrectomy for gastric cancer in the upper third of the stomach: a review of the literature published from 2000 to 2014. Surg Today 2016;46:517-27. [Crossref] [PubMed]
- Hiki N, Nunobe S, Kubota T, et al. Function-preserving gastrectomy for early gastric cancer. Ann Surg Oncol 2013;20:2683-92. [Crossref] [PubMed]
- Koeda K, Nishizuka S, Wakabayashi G. Minimally invasive surgery for gastric cancer: the future standard of care. World J Surg 2011;35:1469-77. [Crossref] [PubMed]
- Takayama T, Matsumoto S, Wakatsuki K, et al. Novel laparoscopic procedure for treating proximal early gastric cancer: laparoscopy-assisted pylorus-preserving nearly total gastrectomy. Surg Today 2014;44:2332-8. [Crossref] [PubMed]
- Kamikawa Y, Kobayashi T, Ueyama S, et al. A new antireflux procedure in esophagogastrostomy after proximal gastrectomy. Shokakigeka 2001;24:1053-60.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Shibao K, Higure A, Yamaguchi K. Access Device 2: Multi-Channel Port. In: Mori T, Dapri G. editors. Reduced Port Laparoscopic Surgery. New York: Springer, 2014:44-56.
- Shibao K, Sato N, Higure A, et al. A new oval multichannel port to facilitate reduced port distal gastrectomy. Minim Invasive Ther Allied Technol 2015;24:135-40. [Crossref] [PubMed]
- Omori T, Oyama T, Akamatsu H, et al. Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg Endosc 2011;25:2400-4. [Crossref] [PubMed]
- Muraoka A, Kobayashi M, Kokudo Y. Laparoscopy-Assisted Proximal Gastrectomy with the Hinged Double Flap Method. World J Surg 2016;40:2419-24. [Crossref] [PubMed]
- Gagner M, Garcia-Ruiz A. Technical aspects of minimally invasive abdominal surgery performed with needlescopic instruments. Surg Laparosc Endosc 1998;8:171-9. [Crossref] [PubMed]
- Tagaya N, Abe A, Kubota K. Needlescopic surgery for liver, gallbladder and spleen diseases. J Hepatobiliary Pancreat Sci 2011;18:516-24. [Crossref] [PubMed]
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Shibao K, Murayama R, Hirata K. Advanced technique of reduced-port proximal gastrectomy for gastric cancer. Ann Laparosc Endosc Surg 2017;2:69.