Laparoscopic proximal gastrectomy for upper third early gastric cancer
Introduction
The rates of early gastric cancer and of upper third gastric cancer have increased over time (1). Proximal gastrectomy for upper third early gastric cancer theoretically allows the storage, digestion, and absorption of food and prevents anemia. The oncologic safety of proximal gastrectomy for upper third early gastric cancer is considered comparable to that of total gastrectomy. However, the widespread adoption of proximal gastrectomy has been limited by the high rates of reflux esophagitis and associated anastomotic stenosis, with 16.2–61.5% of patients reported to experience at least one of these complications after proximal gastrectomy (2). Its minimal invasiveness and function preservation make laparoscopic proximal gastrectomy theoretically superior to open proximal gastrectomy, open total gastrectomy, and laparoscopic total gastrectomy in the treatment of patients with upper third early gastric cancer.
Studies comparing clinical outcomes of laparoscopic proximal gastrectomy followed by esophagogastrostomy with laparoscopic total gastrectomy in patients with upper third early gastric cancer found that the former group had a higher rate of reflux esophagitis (3,4). Therefore, a procedure that included laparoscopic proximal gastrectomy followed by double tract reconstruction has been developed to reduce the risks of reflux esophagitis.
Patient selection and workup
All patients are evaluated by gastroscopy and computed tomography preoperatively. Patients are indicated for laparoscopic proximal gastrectomy if they have a single lesion located in the proximal stomach (upper one third) and confined to the mucosa or submucosa (cT1a or T1b); if there is no evidence of metastatic enlarged lymph nodes (LNs) in LN areas 4d, 5, 6, and, 10 and of distant metastases; and if the lesion is beyond the absolute indications for endoscopic submucosal dissection.
Preoperative preparation
Endoscopic clipping at the proximal and distal sides of the lesion is recommended to determine the resection line of the stomach.
Procedure (Figure 1)

Laparoscopic proximal gastrectomy
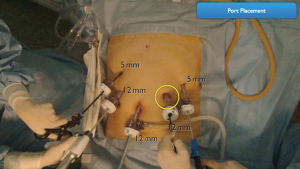
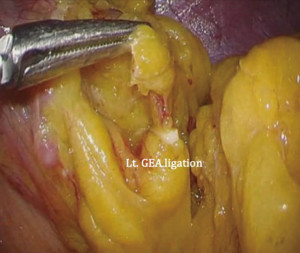
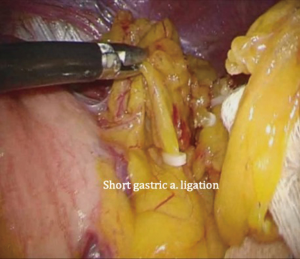
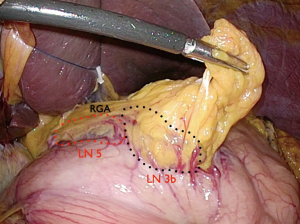
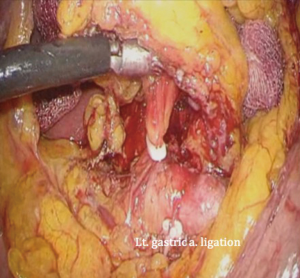
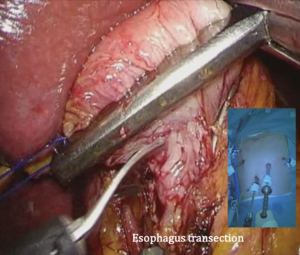
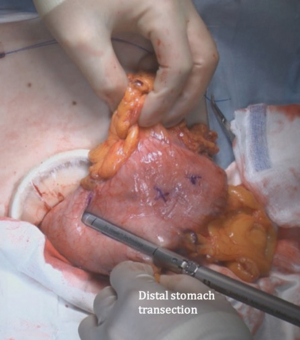
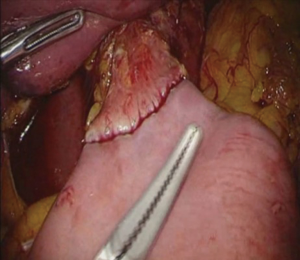
The patient is placed in the reverse Trendelenburg position under general anesthesia. The operator and laparoscopist stand on the right side of the patient, and the first assistant on the left side. Five working ports are created: a 12 mm umbilical port; 5 mm right and left ports along the mid-axillary line below the costal margin; and 12 mm right and left ports along the mid-clavicular line 2 cm above the umbilicus (Figure 2). A combined suture technique is used to lift the falciform ligament and the left lobe of the liver. The operator starts partial omentectomy from the middle of the transverse colon, about 4 cm from the gastroepiploic vessel arcade, continuing toward the spleen. The left gastroepiploic artery and vein are ligated at their roots using hemoclips, but at positions distal to the splenic branch to prevent infarct of the lower pole of the spleen (Figure 3). The short gastric artery and veins are ligated and cut as much as possible (Figure 4), a procedure that includes dissection of LN stations 4sb and 4sa. The omentectomy is continued toward the right side of the patient, up to the transitional zone of LN stations 4d and 6, taking care to preserve the right gastroepiploic artery and vein. The peritoneum is detached along the upper border of the pancreas to mobilize the distal stomach. On the lesser curvature of the stomach, the operator identifies the boundary between LNs 3a (located along the branches of the left gastric artery) and 3b (located along the second branch and distal part of the right gastric artery). LN 3a should be dissected, while LN 3b should be preserved (Figure 5). The pyloric and hepatic branches are usually preserved to prevent delayed gastric emptying. The stomach is transected at this time or later, depending on tumor location. If the tumor is located in the cardia, the stomach should be transected before the celiac axis LNs are dissected, thereby exposing the operative field for LN dissection. If the tumor is located in the high body, however, extracorporeal transection may be possible after palpating the preoperative endoscopic clip. LNs 7, 8a, and 9 are dissected, during which the coronary vein and left gastric artery are ligated and cut (Figure 6). The operator continues to dissect LN 11p along the splenic artery and vein up to the splenic hilum, including LN 11d at the distal splenic artery. The esophagogastric junction is exposed, and LNs 1 and 2 are dissected. If the tumor has invaded the esophagus, as in Siewert type II gastroesophageal junction cancer, lower mediastinal LN dissection should be performed. The distal esophagus is transected using linear staplers or proximal (LapJack; Eterne, Seoul, Korea) and distal (Endo-bulldog; B. Braun Melsungen AG, Melsungen, Germany) laparoscopic purse-string clamps, which are applied to prevent spillage from the stomach (Figure 7). If circular staples are used, purse-string sutures are made using a straight needle with prolene 2-0. LN 2 and any remaining LNs in area 4sa are dissected in a downstream manner. A 4 cm mini-laparotomy is made by extending the left 12 mm trocar site, followed by extraction of the resected specimen if the stomach had already been transected. Alternatively, the upper and middle parts of the stomach are obtained, followed by checking the distal resection margin with clips and transection of the stomach with linear staplers (Figure 8).
Reconstructions
Direct esophagogastrostomy
Although easy to perform, direct esophagogastrostomy may induce severe esophageal reflux in some patients. Adjunct procedures to enhance the safety of direct esophagogastrostomy include antireflux procedures, overlap anastomosis, and preservation of the lower esophageal sphincter (6-8).
Jejunal interposition
Jejunal interposition is relatively complex compared with direct esophagogastrostomy or double tract reconstruction. This procedure requires the construction of a pedicle of the jejunal limb and three anastomoses including an esophagojejunostomy, a jejunogastrostomy, and a jejunojejunostomy (9-11).
Double tract reconstruction
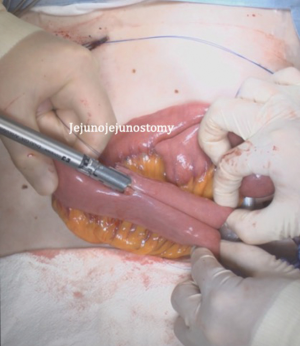
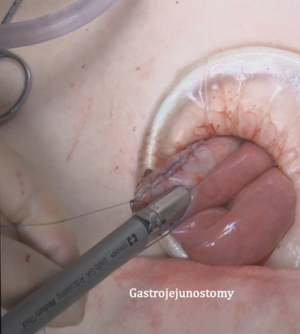
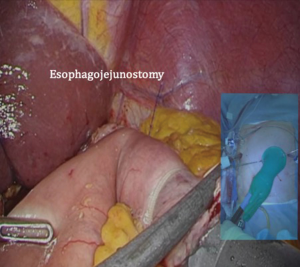
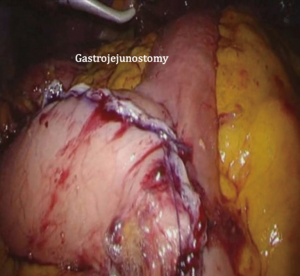
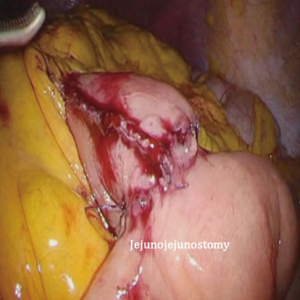
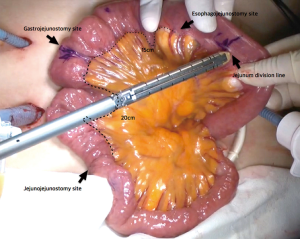
After transection of the stomach, a single stitch is made on the fusion stapler line of the remnant stomach and the latter is put back into the abdominal cavity. The anvil of the circular stapler is introduced into the abdominal cavity through mini-laparotomy, followed by pneumoperitoneum. Using a laparoscopic anvil clamp, the operator inserts the anvil head into the esophageal stump intracorporeally and ties the purse-string sutures (12). Subsequently, the jejunum is pulled out via the mini-laparotomy and the operator marks the sites of esophagojejunostomy, gastrojejunostomy, and jejunojejunostomy (Figure 9). The order of the anastomoses depends on the surgeon’s preference. Using linear staplers, the jejunum is transected, and a side-to-side jejunojejunostomy is created 20 cm distal from the gastrojejunostomy (Figure 10). The jejunojejunal mesenteric defect is closed to prevent internal herniation of the small bowel. A side-to-side gastrojejunostomy 15 cm distal to the esophagojejunostomy is made in an extracorporeal manner using a linear stapler. The linear stapler is inserted into the posterior wall of the stomach and the Roux limb (Figure 11). A circular stapler is subsequently inserted into the Roux limb, and an end-to-side esophagojejunostomy is made intracorporeally (Figure 12). The jejunal stump is closed with a linear stapler, completing all three anastomoses (Figures 13,14,15).
Role of team members
Each surgical team consists of an operator, a first assistant, and a laparoscopist. If reduced or single port laparoscopic proximal gastrectomy is feasible, the first assistant or laparoscopist may be replaced by a scope holder.
Postoperative management
Patients are allowed sips of water, a semi-liquid diet, and a soft blended diet on postoperative days 2, 3, and 4, respectively, followed by removal of the J-P drain. Patients are usually discharged from the hospital on postoperative day 5 or 6, unless they experience fever, abdominal pain, or abnormal laboratory test results.
Tips, tricks, and pitfalls
The 2014 Japanese Gastric Cancer Treatment Guidelines (ver. 4) recommend that patients with proximal tumors undergo proximal gastrectomy when more than half the distal stomach can be preserved (13). Transection of the stomach at the junction of LNs 3a and 3b can result in preservation of more than half the distal stomach.
The Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) 05: phase III multicenter prospective randomized clinical trial of laparoscopic proximal gastrectomy with double tract reconstruction and laparoscopic total gastrectomy for upper early gastric cancer.
Although laparoscopic proximal gastrectomy with double tract reconstruction was reported to be superior to laparoscopic total gastrectomy in patients with upper third early gastric cancer, these findings were based on retrospective results. To our knowledge, no prospective randomized controlled trial has compared these outcomes. We therefore designed KLASS-05, a randomized clinical trial comparing laparoscopic proximal gastrectomy plus double tract reconstruction with laparoscopic total gastrectomy alone. The results of KLASS-05 may help surgeons determine the optimal surgical strategy for patients with proximal early gastric cancer.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.03.01). HHK serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jul 2016 to Jun 2018. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer 2011;11:69-77. [Crossref] [PubMed]
- Ahn SH, Lee JH, Park DJ, et al. Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer 2013;16:282-9. [Crossref] [PubMed]
- Masuzawa T, Takiguchi S, Hirao M, et al. Comparison of perioperative and long-term outcomes of total and proximal gastrectomy for early gastric cancer: a multi-institutional retrospective study. World J Surg 2014;38:1100-6. [Crossref] [PubMed]
- Nozaki I, Hato S, Kobatake T, et al. Long-term outcome after proximal gastrectomy with jejunal interposition for gastric cancer compared with total gastrectomy. World J Surg 2013;37:558-64. [Crossref] [PubMed]
- Park DJ, Lee Y, Ahn SH, et al. Laparoscopic proximal gastrectomy with double tract reconstruction. Asvide 2017;4:174. Available online: http://www.asvide.com/articles/1482
- Uyama I, Sugioka A, Matsui H, et al. Laparoscopic side-to-side esophagogastrostomy using a linear stapler after proximal gastrectomy. Gastric Cancer 2001;4:98-102. [Crossref] [PubMed]
- Sakuramoto S, Yamashita K, Kikuchi S, et al. Clinical experience of laparoscopy-assisted proximal gastrectomy with Toupet-like partial fundoplication in early gastric cancer for preventing reflux esophagitis. J Am Coll Surg 2009;209:344-51. [Crossref] [PubMed]
- Kim DJ, Lee JH, Kim W. Lower esophageal sphincter-preserving laparoscopy-assisted proximal gastrectomy in patients with early gastric cancer: a method for the prevention of reflux esophagitis. Gastric Cancer 2013;16:440-4. [Crossref] [PubMed]
- Nomura E, Lee SW, Kawai M, et al. Functional outcomes by reconstruction technique following laparoscopic proximal gastrectomy for gastric cancer: double tract versus jejunal interposition. World J Surg Oncol 2014;12:20. [Crossref] [PubMed]
- Uyama I, Sugioka A, Fujita J, et al. Completely laparoscopic proximal gastrectomy with jejunal interposition and lymphadenectomy. J Am Coll Surg 2000;191:114-9. [Crossref] [PubMed]
- Kinoshita T, Gotohda N, Kato Y, et al. Laparoscopic proximal gastrectomy with jejunal interposition for gastric cancer in the proximal third of the stomach: a retrospective comparison with open surgery. Surg Endosc 2013;27:146-53. [Crossref] [PubMed]
- Ahn SH, Jung DH, Son SY, et al. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer 2014;17:562-70. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
Cite this article as: Park DJ, Lee Y, Ahn SH, Kim HH. Laparoscopic proximal gastrectomy for upper third early gastric cancer. Ann Laparosc Endosc Surg 2017;2:80.