Laparoscopic right hepatectomy for living donation
Introduction
Living donor liver transplantation has become a widely accepted alternative to cadaveric transplantation (1,2). Such living donor grafts provide similar or better short-term graft function and long-term survival rates when compared with cadaveric liver grafts, especially in children (3,4). This procedure has the advantages to shorten waiting list time and to minimize cold ischemia time. However, the surgical risk brought upon donors remains the most important concern (4,5). In the setting of adult to adult liver transplantation, the development of major liver resection has been slow, due to an increased technical complexity and significant donor morbidity (near 40%) (6,7).
The rising of laparoscopic liver resections, initially for tumors, has been limited in live donor hepatectomy (LDH), due to initial uncertainty on its technical feasibility and safety, especially for major hepatectomy. Lately, this approach has gained more acceptance, demonstrating significant advantages such as decreased postoperative morbidity (8-11) shorter hospital stay (11,12), and reduced global costs (13). Hence, laparoscopic approach is now considered as the standard technique for several procedures, such as left lateral sectionectomy (14,15).
In the meantime, laparoscopic living donor nephrectomy has become the standard approach for renal transplantation, exhibiting reduced morbidity and faster recovery, which led to increased donation rates (16). The application to liver donation for transplantation may bring the same benefits and help to minimize surgical trauma from the large abdominal incision. In this setting, laparoscopic left lateral sectionectomy for living donation has gained wide approval and is now considered as the standard technique in expert teams (17,18). The development of laparoscopic live donor major hepatectomy, despite slower, seems promising, due to accumulating experience and constant refinements.
The objective of this article is to review and discuss every aspect of laparoscopic live donor right hepatectomy, from surgical indication to technical aspects.
The development of laparoscopic right hepatectomy for living donation: initial experience, evolution and current position
Initial experience of live donor right hepatectomy
The first LDH for a pediatric transplantation was reported in 1989 and 1990 (1,2). Since then, LDH has developed mostly in Eastern countries, owing to deceased donor scarcity. The first case of a right liver donation for adult liver transplant was then described in 1994 by Yamaoka et al. (19).
In the very first developmental stage, LDLT was considered with great enthusiasm, and accounted for 10% of the total amount of the United States liver transplantations in 2001 (20). Further reports of an approximate 0.2% mortality (4) and 40% complication rate (7,21) associated to open right LDH were published. Reported complications included biliary leak, sepsis or pulmonary complications (22,23). These risks, associated with reported psychological difficulties (7) may have dissuaded donors and troubled general opinion, as well as medical teams. While LT from available deceased donors remains an alternative in Western countries, these potential risks led to some reticence among medical teams and to a significant decrease in the number of procedures, which now account for 4% of the total liver transplantations in the United States (20).
In this context, several expert teams recommended the laparoscopic approach for LDH as a way to reduce donor morbidity.
The first cases of laparoscopic LDH have been reported by in 2002 (24). Full laparoscopic left (25,26) and right (27-29) hepatectomy were reported for adult-to-adult transplant afterwards. Besides the pure “full” or “totally” laparoscopic approach, the hand assisted laparoscopic technique and the laparoscopic assisted or “hybrid” technique (30,31) were described. There is currently no evidence in the literature demonstrating the superiority of one technique over the others (32).
Evolution and learning curve of laparoscopic right LDH
The diffusion of the laparoscopic approach for right LDH was slower than the open technique, owing to several concerns: (I) the overall technically demanding technique; (II) concerns regarding donor safety, especially with the risk of hemorrhage during surgery; (III) concerns regarding the graft safety and prolonged warm ischemia time due to its extraction; and (IV) the lack of comparative studies with the standard open approach.
Indeed, laparoscopic major liver resection for LDLT requires expertise in both LDLT and laparoscopic liver surgery. Several reports demonstrated that a learning curve of minimum 15–60 procedures was necessary to reach optimal results (33,34). Moreover, the small proportion of major hepatectomy for LDLT performed annually, especially in Western countries (35), contributes to the slow progression of the technique.
However, there is now accumulating evidence that laparoscopic right hepatectomy for classical liver resection demonstrates better surgical outcomes than the open approach, with reduced postoperative morbidity (8,10,11), shorter hospital stay (11,12), or improved cost effectiveness (13).
The technical improvements and growing expertise of the last two decades in laparoscopic liver resection allowed reducing blood loss and transfusion rates compared to patients undergoing open liver resection (36,37). For example, more effective hemostasis devices, magnified laparoscopic vision with 30° laparoscopes, reverse Trendelenburg positioning in order to reduce hepatic backflow, or pneumoperitoneum increase (38,39) have been described to improve intraoperative bleeding management. Moreover, the surgical community benefits from accumulating experience on laparoscopic left lateral sectionectomy for living donation, which had gained broad acceptance through standardization of the technique and excellent reported outcomes (17,18).
In the setting of fully laparoscopic LDH, several authors have raised some concerns for graft integrity during its extraction in a plastic bag through a small incision and the possible associated prolonged warm ischemia time (40). Our team demonstrated that even though laparoscopy-retrieved grafts undergo increased warm ischemia time, this had no impact on graft-related complications or survival (41).
Since a randomized controlled trial is very unlikely in this setting, accumulating evidence on the benefits of laparoscopic approach in LDLT rely mostly on retrospective or case-matched reports, but definitely contribute to the global acceptance and development of the technique. To date, 167 cases of laparoscopic right hepatectomy for adult-to-adult LDLT have been reported between 2006 and 2014, mostly through case reports, case series or case match series (39).
Current position
The second international consensus on laparoscopic liver resection (Morioka, Japan) concluded that left lateral sectionectomy for pediatric transplant brought the advantages of minimally-invasive surgery, associated to a safety level not different from open surgery (42). In addition, recent large cohorts published by expert teams show excellent outcomes and recommend the laparoscopic approach as a gold standard for left lateral section retrieval for LDLT (17,18). However, in the setting of adult-to-adult LDH, the experts recommended the creation of a registry to evaluate the benefit/risk ratio, and the Jury did not recommend broad introduction since its safety was not proven yet.
Undoubtedly, major laparoscopic LDH requires a high level of technical skills and has a steep learning curve, restraining its practice to expert teams. However, growing surgeon experience, continuous technical refinements and increasing standardization efforts participate in its diffusion an acceptance.
Donor evaluation process
The selection of an eligible donor means the evaluation of, in one hand, the technical feasibility, and in the other hand, the operative risk for the donor. Such issues imply an extensive workup procedure, including extensive detection of any medical contra-indication, donor psychological assessment, and suitability of the intended graft in terms of anatomy, volume and function.
Donor operative risk evaluation
Currently, there is no consensus for donor age, and we rely more on physiological age than biological age. However, an upper age limit is arbitrarily set at 55 in many centers. Every eligible donor has to be informed about the risks involved in the operation, and the recipient mortality estimation (5%), together with donor mortality (0.2%) and morbidity rates (up to 40%), have to be explained. The innovative nature of the pure laparoscopic technique and its impact on these risks has to be underlined.
An extensive medical history interrogation including in particular personal and/or familial history of diabetes mellitus, cardiovascular diseases, pulmonary disease, malignancy, psychological disorders, alcohol consumption and smoking, deep vein thrombosis risk factors (oral contraception use for women) should be conducted. This must be completed by a complete physical examination including BMI and arterial blood pressure measurements. Biological tests include, besides classical blood hematology and biochemistry, complete glycemic and lipidic profile, common viral serology screening (HIV, hepatitis B and C, CMV, VZV), and, in our center, an extensive research for coagulation disorders (factor V Leiden, factor II, antithrombin III, antiphospholipid antibodies, protein C, protein S). Specific markers for malignancy can be added in case of any clinical suspicion.
A psychiatrist should conduct a complete psychological evaluation and the donor case has to be presented for approval by an ethical committee. Last, according to the French Law, all donors give their informed consent, which is recorded by a Judge of the Civilian Court.
Technical feasibility: graft assessment
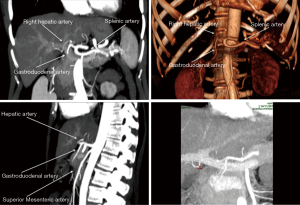
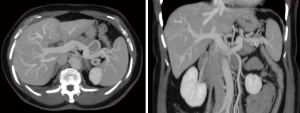
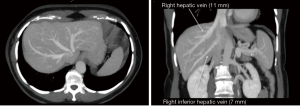
The graft assessment workup procedure includes multiple imaging evaluations by ultrasound, CT-scan and MRI cholangiography together. CT-scan with 3D vascular reconstruction is mandatory for arterial mapping. The course and size of hepatic artery and detection of anatomical variations such as a right hepatic artery from the superior mesenteric artery or left hepatic artery arising from the left gastric artery are notified (Figure 1). Origin of segment IV hepatic artery should be outlined (from left or right hepatic artery). Portal veins anatomy is also evaluated (Figure 2), in order to identify division abnormalities such as portal trifurcation. Particular attention is paid to hepatic venous drainage (Figure 3).
CT volumetric measurement of the liver remnant and the intended graft should be performed in order to have a safety limit for donor of 30–35% remnant volume, and to ensure of the 0.8% graft to body weight ratio necessary for the recipient (43-46).
At last, we require a magnetic resonance cholangiography, which is nowadays the best imaging to detect biliary anatomy abnormalities (Figure 4). This investigation is fundamental to search for variations as a right posterior or anterior sectorial duct joining the left hepatic duct; such a situation represents a formal contra-indication to the intervention. Position of left hepatic duct division and site of segment IV duct joining should be recognized as well.
We do not routinely perform liver biopsy, nor ERCP or arteriography in our center.
Finally, the decision of acceptance to perform a living donor right hepatectomy is validated in a multidisciplinary expert reunion, where all potential medical contra-indication are considered, and where suitability of the intended graft in terms of anatomy and volume are closely examined.
Surgical technique (Figure 5)

To enhance the donor safety, two senior surgeons may ideally perform this intervention. Any incident that might compromise donor safety or graft integrity should lead to prompt conversion. In our team, we defined these events or criteria of conversion as following: significant bleeding, failure to accurately recognize bile duct anatomy, any vessel injury, and poor exposure leading to slow progression during parenchymal transection. The operation is divided in three main steps: right pedicle dissection, parenchymal transection including bile duct division, and graft extraction. Usually, these steps are performed in rotation between the two surgeons, one performing the pedicle dissection and the other doing the parenchymal transection and graft removal.
Installation
The donor is placed in French position, legs apart. Devices to prevent hypothermia (warming coverage) and deep vein thrombosis (compression stockings) are systematically used.
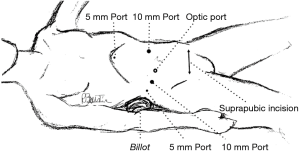
A carbon dioxide pneumoperitoneum is created and maintained at 12 mmHg pressure. Five ports are inserted as shown in Figure 6. The optic port is placed 2 to 3 cm above and laterally to the umbilicus to avoid any tangential vision to the right liver.
A 30° laparoscope is mandatory, in order to have an optimal vision of every parcel of the abdominal cavity, especially to nicely visualize the hepatic vein and to avoid any discomfort in case of tangential dissection area.
Access to the right liver graft and right pedicle preparation
After a general inspection of the liver and the abdominal cavity, the cystic artery and duct are clipped and divided. After opening the hepatic pedicle, the right hepatic artery and right portal vein are dissected free and taped. Cholecystectomy is performed and the round and falciform ligaments are disconnected.
The right part of segment I is separated to expose the anterior side of the inferior vena cava (IVC), with dividing of the small accessory veins arising from segment I. This part of the dissection is performed as high as possible but without reaching the right hepatic vein. No hanging maneuver is performed and we do not mobilize the right liver at that time.
The right portal vein and hepatic artery are briefly clamped in order to visualize the main portal scissure. The middle hepatic vein, which is kept with the left liver, is then identified with intra-operative ultrasound.
Parenchymal transection
The harmonic scalpel is used for the capsule incision and the superficial part of the transection (no more than 1 cm deep in the parenchyma), and we prefer the ultrasonic dissector for deeper transection.
The parenchyma transection follows the Cantlie line, the upward trajectory of the IVC, and the middle hepatic vein, which is previously located using ultrasound. This laparoscopic visualization from below allows the surgeon to follow the IVC throughout the transection, as previously described by our team as the “inferior approach” (48).
The parenchyma is thus divided step by step and the encountered vascular pedicles are identified before clipping and dividing. Vascular elements larger than 2 mm, such as pedicles assigned to segment 4, are dissected free using ultrasonic dissector, taped using PTFE tapes in order to nicely expose both sides of the pedicle that need to be transected, closed using secured Hem-o-lock® clips (non absorbable polymer vascular clips, Weck, Teleflex Medical, Limerick, Pennsylvania, USA), and divided.
Bleeding is controlled only by using bipolar cautery for minor vessels, and clips for larger vascular structures. No vascular clamping is used in order to minimize both donor and graft damage. A transient increase in pneumoperitoneum pressure up to 16 mmHg can be applied, if well tolerated by the donor, to improve bleeding control.
Right bile duct division
After identification of the right bile duct, an intra-operative cholangiography is performed through the cystic duct. The right bile duct is then divided with scissors at the level of a marker thread. No electric cautery should be applied in this step in order to avoid thermal injury of the biliary duct or the hilar plate. Its distal stump is closed using a secured clip.
End of transection and control of the right hepatic vein
After bile duct division, the parenchyma transection is completed and the right hepatic vein is dissected free, controlled and taped. The right liver is then mobilized by cutting the right triangular ligament. At this stage, the graft is only retained by its vessels.
Graft harvesting
A 10-cm suprapubic incision is made without opening the peritoneum. This allows the insertion of a large bag through a 15-mm port. The right hepatic artery is closed with a secured clip, then the right portal and hepatic veins are closed using a one-sided stapler and then divided. The right liver graft is placed in the bag and removed through the suprapubic incision without muscle section.
The graft is immediately weighed and perfused with a cold preservation solution through the left portal vein, initiating end of warm ischemia time (usually less than 10 min) and beginning of cold ischemia time. The bile ducts are washed with the same preservation solution.
Graft preparation also includes venous outflow optimization. The veins from segments 5 and 8 may be reconstructed using an allogeneic iliac graft from tissue bank. The right hepatic vein stump can be enlarged with a cuff tailored in an iliac conduit from tissue bank.
After re-insufflation, hemostasis and biliostasis are checked. CO2 pneumoperitoneum is completely evacuated to reduce postoperative pain.
Postoperative care
We systematically use prophylactic dose of low molecular weight heparin and compression stockings from postoperative day one for the prevention of deep vein thrombosis Proton pump inhibitors are used systematically to prevent gastric ulcer. No postoperative gastric tube is retained.
Oral intake is allowed in the evening of the procedure, and early mobilisation is encouraged on postoperative day one. Particular attention is paid to postoperative pain and appropriate painkillers are prescribed; their efficiency and necessity are reevaluated daily.
Clinical features and biological tests are closely monitored every day; particular attention is paid to pulmonary examination and any sign of pulmonary embolism is carefully sought. Biological liver function is daily assessed by biochemical tests including prothrombin time and serum bilirubin. Any clinical or biological sign of general or liver-related complication is reported.
Hand-assisted and hybrid techniques
Unlike pure laparoscopic approach, hand-assisted procedure uses a hand port through a right subcostal short incision to facilitate the operation, especially retrohepatic IVC dissection (49).
In hybrid procedure, pedicular dissection and liver mobilization are performed using laparoscopy while liver parenchyma transection and specimen extraction are performed through a short (midline or subcostal) incision.
These methods have been described and compared previously (31,50,51). To date, there is no evidence showing the superiority of one technique over the others and the choice of the technique depends on the surgeons’ experience.
Conclusions
In summary, the right liver can be harvested in a living donor through pure laparoscopic approach in highly experienced centers. We believe that its benefits may increase the potential donation rate, based on a growing global acceptance. Accumulating case series report its technical feasibility and comparable outcomes to conventional approach. However, careful validation through larger series is mandatory before laparoscopic right hepatectomy for live donation may be ready for wider diffusion and acceptance. The question of donor safety remains the main concern of live donor liver transplantation, and improved reproducibility of the procedure is needed through standardization and registry studies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giovanni Battista Levi Sandri) for the series “Laparoscopic Liver Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.02.13). The series “Laparoscopic Liver Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raia S, Nery JR, Mies S. Liver transplantation from live donors. Lancet 1989;2:497. [Crossref] [PubMed]
- Strong RW, Lynch SV, Ong TH, et al. Successful liver transplantation from a living donor to her son. N Engl J Med 1990;322:1505-7. [Crossref] [PubMed]
- Lo CM. Complications and long-term outcome of living liver donors: a survey of 1,508 cases in five Asian centers. Transplantation 2003;75:S12-5. [Crossref] [PubMed]
- Cheah YL, Simpson MA, Pomposelli JJ, et al. Incidence of death and potentially life-threatening near-miss events in living donor hepatic lobectomy: a world-wide survey. Liver Transpl 2013;19:499-506. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Marsh JW, Gray E, Ness R, et al. Complications of right lobe living donor liver transplantation. J Hepatol 2009;51:715-24. [Crossref] [PubMed]
- Abecassis MM, Fisher RA, Olthoff KM, et al. Complications of Living Donor Hepatic Lobectomy--A Comprehensive Report. Am J Transplant 2012;12:1208-17. [Crossref] [PubMed]
- Kim SJ, Jung HK, Lee DS, et al. The comparison of oncologic and clinical outcomes of laparoscopic liver resection for hepatocellular carcinoma. Ann Surg Treat Res 2014;86:61-7. [Crossref] [PubMed]
- Mirnezami R, Mirnezami AH, Chandrakumaran K, et al. Short- and long-term outcomes after laparoscopic and open hepatic resection: systematic review and meta-analysis. HPB 2011;13:295-308. [Crossref] [PubMed]
- Tranchart H, Di Giuro G, Lainas P, et al. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc 2010;24:1170-6. [Crossref] [PubMed]
- López-Ben S, Palacios O, Codina-Barreras A, et al. Pure laparoscopic liver resection reduces surgical site infections and hospital stay. Results of a case-matched control study in 50 patients. Langenbecks Arch Surg 2014;399:307-14. [Crossref] [PubMed]
- Truant S, Bouras AF, Hebbar M, et al. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc 2011;25:3668-77. [Crossref] [PubMed]
- Medbery RL, Chadid TS, Sweeney JF, et al. Laparoscopic vs open right hepatectomy: a value-based analysis. J Am Coll Surg 2014;218:929-39. [Crossref] [PubMed]
- Chang S, Laurent A, Tayar C, et al. Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg 2007;94:58-63. [Crossref] [PubMed]
- Dokmak S, Raut V, Aussilhou B, et al. Laparoscopic left lateral resection is the gold standard for benign liver lesions: a case-control study. HPB 2014;16:183-7. [Crossref] [PubMed]
- Schweitzer EJ, Wilson J, Jacobs S, et al. Increased rates of donation with laparoscopic donor nephrectomy. Ann Surg 2000;232:392-400. [Crossref] [PubMed]
- Soubrane O, de Rougemont O, Kim KH, et al. Laparoscopic Living Donor Left Lateral Sectionectomy: A New Standard Practice for Donor Hepatectomy. Ann Surg 2015;262:757-61; discussion 761-3. [Crossref] [PubMed]
- Scatton O, Katsanos G, Boillot O, et al. Pure laparoscopic left lateral sectionectomy in living donors: from innovation to development in France. Ann Surg 2015;261:506-12. [Crossref] [PubMed]
- Yamaoka Y, Washida M, Honda K, et al. Liver transplantation using a right lobe graft from a living related donor. Transplantation 1994;57:1127-30. [Crossref] [PubMed]
- Organ Procurement and Transplantation Network. Transplants by donor type. Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data
- Ghobrial RM, Freise CE, Trotter JF, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology 2008;135:468-76. [Crossref] [PubMed]
- Belghiti J, Liddo G, Raut V, et al. “Inherent limitations” in donors: control matched study of consequences following a right hepatectomy for living donation and benign liver lesions. Ann Surg 2012;255:528-33. [Crossref] [PubMed]
- Dondero F, Taillé C, Mal H, et al. Respiratory complications: a major concern after right hepatectomy in living liver donors. Transplantation 2006;81:181-6. [Crossref] [PubMed]
- Cherqui D, Soubrane O, Husson E, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet 2002;359:392-6. [Crossref] [PubMed]
- Samstein B, Cherqui D, Rotellar F, et al. Totally laparoscopic full left hepatectomy for living donor liver transplantation in adolescents and adults. Am J Transplant 2013;13:2462-6. [Crossref] [PubMed]
- Troisi RI, Wojcicki M, Tomassini F, et al. Pure laparoscopic full-left living donor hepatectomy for calculated small-for-size LDLT in adults: proof of concept. Am J Transplant 2013;13:2472-8. [Crossref] [PubMed]
- Soubrane O, Perdigao Cotta F, Scatton O. Pure laparoscopic right hepatectomy in a living donor. Am J Transplant 2013;13:2467-71. [Crossref] [PubMed]
- Takahara T, Wakabayashi G, Hasegawa Y, et al. Minimally invasive donor hepatectomy: evolution from hybrid to pure laparoscopic techniques. Ann Surg 2015;261:e3-4. [Crossref] [PubMed]
- Han HS, Cho JY, Yoon YS, et al. Total laparoscopic living donor right hepatectomy. Surg Endosc 2015;29:184. [Crossref] [PubMed]
- Koffron AJ, Kung R, Baker T, et al. Laparoscopic-assisted right lobe donor hepatectomy. Am J Transplant 2006;6:2522-5. [Crossref] [PubMed]
- Baker TB, Jay CL, Ladner DP, et al. Laparoscopy-assisted and open living donor right hepatectomy: a comparative study of outcomes. Surgery 2009;146:817-23; discussion 823-5. [Crossref] [PubMed]
- Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg 2013;257:205-13. [Crossref] [PubMed]
- Vigano L, Laurent A, Tayar C, et al. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg 2009;250:772-82. [Crossref] [PubMed]
- Cai X, Li Z, Zhang Y, et al. Laparoscopic liver resection and the learning curve: a 14-year, single-center experience. Surg Endosc 2014;28:1334-41. [Crossref] [PubMed]
- Dutkowski P, De Rougemont O, Müllhaupt B, et al. Current and future trends in liver transplantation in Europe. Gastroenterology 2010;138:802-9.e1-4.
- Fancellu A, Rosman AS, Sanna V, et al. Meta-analysis of trials comparing minimally-invasive and open liver resections for hepatocellular carcinoma. J Surg Res 2011;171:e33-45. [Crossref] [PubMed]
- Xiong JJ, Altaf K, Javed MA, et al. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol 2012;18:6657-68. [Crossref] [PubMed]
- Costi R, Scatton O, Haddad L, et al. Lessons learned from the first 100 laparoscopic liver resections: not delaying conversion may allow reduced blood loss and operative time. J Laparoendosc Adv Surg Tech A 2012;22:425-31. [Crossref] [PubMed]
- Cauchy F, Schwarz L, Scatton O, et al. Laparoscopic liver resection for living donation: where do we stand? World J Gastroenterol 2014;20:15590-8. [Crossref] [PubMed]
- Borle DP, Bharathy KG, Kumar S, et al. Laparoscopic living donor left hepatectomy: donor safety remains the overriding concern. Am J Transplant 2014;14:735. [Crossref] [PubMed]
- Soubrane O, Cherqui D, Scatton O, et al. Laparoscopic left lateral sectionectomy in living donors: safety and reproducibility of the technique in a single center. Ann Surg 2006;244:815-20. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Redvanly RD, Nelson RC, Stieber AC, et al. Imaging in the preoperative evaluation of adult liver-transplant candidates: goals, merits of various procedures, and recommendations. AJR Am J Roentgenol 1995;164:611-7. [Crossref] [PubMed]
- Lo CM, Fan ST, Liu CL, et al. Minimum graft size for successful living donor liver transplantation. Transplantation 1999;68:1112-6. [Crossref] [PubMed]
- Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation 1999;67:321-7. [Crossref] [PubMed]
- Sakamoto S, Uemoto S, Uryuhara K, et al. Graft size assessment and analysis of donors for living donor liver transplantation using right lobe. Transplantation 2001;71:1407-13. [Crossref] [PubMed]
- Goumard C, Soubrane O, Brustia R, et al. Totally laparoscopic right hepatectomy for living donation. Asvide 2017;4:172. Available online: http://www.asvide.com/articles/1480
- Soubrane O, Schwarz L, Cauchy F, et al. A Conceptual Technique for Laparoscopic Right Hepatectomy Based on Facts and Oncologic Principles: The Caudal Approach. Ann Surg 2015;261:1226-31. [Crossref] [PubMed]
- Ha TY, Hwang S, Ahn CS, et al. Role of hand-assisted laparoscopic surgery in living-donor right liver harvest. Transplant Proc 2013;45:2997-9. [Crossref] [PubMed]
- Park JI, Kim KH, Lee SG. Laparoscopic living donor hepatectomy: a review of current status. J Hepatobiliary Pancreat Sci 2015;22:779-88. [Crossref] [PubMed]
- Zhang X, Yang J, Yan L, et al. Comparison of laparoscopy-assisted and open donor right hepatectomy: a prospective case-matched study from china. J Gastrointest Surg 2014;18:744-50. [Crossref] [PubMed]
Cite this article as: Goumard C, Soubrane O, Brustia R, Scatton O. Laparoscopic right hepatectomy for living donation. Ann Laparosc Endosc Surg 2017;2:81.