Perioperative patient care involved with robotic-assisted bariatric surgery
Introduction
Obesity has become one of many serious public health problems in the United States as obesity rates are seen to be increasing every year (1). It is a well-known fact that obesity contributes to various complications such as non-insulin-dependent diabetes, hypertension, and hypercholesterolemia (2).
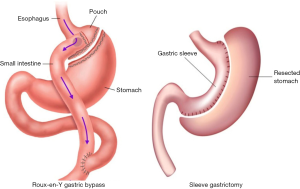
Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are the two most commonly practiced bariatric procedures. In the 1990s, the first laparoscopic RYGB was performed by Wittgrove and Clark (3) and the laparoscopic sleeve gastrectomy was initially performed in 2000 (having been reported by Ren et al.) (4).
Within the last 20 years, the arrival of robotic surgery systems has enabled many complex minimally invasive procedures to be performed in several surgical specialties, including bariatric surgery (5). In September of 2000, Horgan and his colleagues performed the first robot-assisted gastric bypass (6). Since, the robotic-assisted bariatric surgery has gained wider acceptance due to the advantages of using the robot for the bariatric procedure including 3-D vision, freedom of movement of Endowrist instruments, and surgical precision. In the interest of safer care for the patient(s) involved, the operating room requires surgical staff with special robotics training to set up the operative suite and maintain the surgical robotic equipment. Perioperative staff must demonstrate expert execution during setup which includes proficiency connections, positioning, & operations involved in the robotic components.
The purpose of this article is to inform nursing staff of the sequence of events as well as to have special considerations for specific robotic bariatric surgery. It also aims to outline the role of the surgical team members within this particular setting.
Patients and results
From 2009 to 2015, there were 409 patients who underwent robotic-assisted bariatric surgery by a single bariatric surgeon and his team. Of the 409 procedures, 288 were RYGB and 121 were VSG. Of the patients involved their average age was 47.4 and BMI was 53.9 kg/m2. The average operating room times (includes 60–80 mins of anesthesia time and patient positioning time) were 247.7 minutes on RYGB and 142.6 minutes on sleeve gastrectomy. There have not been any complications concerning the patients. Only four patients were converted to open. On average, the post-op hospital stay was 3.7 days.
Methods and procedures
Room setup and patient positioning
The da Vinci Surgical System has three parts which includes surgeon’s console, robotic arms, and video tower. The surgeon controls movement of the robotic arms when seated at the surgeon’s console, both of which are attached to the video tower. One arm holds the camera and scope; the other two—or three if necessary—arms control the robotic instruments. With a thorough understanding of how the robotic system works, surgical staff can provide the most valuable assistance to the surgeon during the procedures and be key players in this cutting edge of robotic surgical care (7).
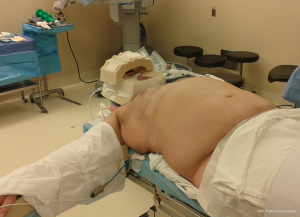
The room organization for both robotic bariatric surgeries are the same, giving special attention to arrange for the sterile field to eventually be positioned on the patient’s left after rotating the operating table about 180 degrees. Prior to transporting the patient to the OR, the nursing staff should confirm that the operating room bed will both accommodate the weight of the obese patient and enter reverse Trendelenburg to the surgeon’s satisfaction. After intubation, the surgical team will move the patient to the edge of the right side of the operating table. This is a crucial step because a flexible retraction instrument will be fixated to the operating table and must be close enough to extend up the patient’s side and reach the liver retractor to suspend the liver in the abdominal cavity. If the patient has excess axillary fat, the circulator will need to remember to pad that area when the instrument is attached to the operating table. The circulator or bed-side assistant will secure a footboard under the patient’s feet, verifying that their feet are flat and legs in a neutral position.
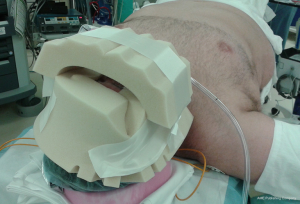
The draw sheet on the bed should be long on the patient’s left side where the patient’s arm will be liberally padded and then tucked. The right arm is left extended on the arm board to allow space for the liver retractor system (Figure 1). The patient will be supine with mild reverse Trendelenburg. Since the robot will be docked above the patient’s head, an arm cradle is used to surround the top and sides of the head. The cradle is secured with a chin strap fashioned out of 3-inch silk tape (Figure 2).
Anesthesia provisions
The surgeon’s assistant should advise the anesthesia provider that the endotracheal tube should be taped to the left side of the patient’s mouth to later accommodate the 34 French bougie dilator (for sleeve gastrectomy) or 34 French gastric lavage tube (for RYGB only). The assistant should also clarify that—in docking the robot over the patient’s head—the patient’s face may not be readily to them during the course of the procedure and to plan accordingly. In urgent circumstances where the anesthesia provider needs access to the patient’s head, the robot may be undocked to better accommodate the necessary interventions. The anesthesia provider should also be aware that the procedure requires rotating the operating table almost 180 degrees, and they will therefore need several IV extensions. After rotating the bed, airway tubing, IV lines, pulse oximeter, and any other monitoring cords will be taped along the patient’s right side down to the feet. After the head wrap is applied, the bougie or gastric lavage tube should be inserted up to about 50 to 55 centimeters from the front teeth.
Operative sequence for robotic RYGB and VSG
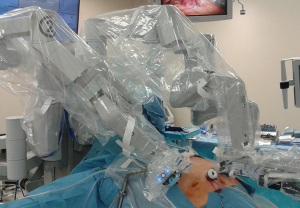
After prepping the skin, draping the patient, and performing the timeout, the surgeon will mark anatomical landmarks and the incision sites for trocars. Figure 3 shows trocar placement for robotic RYGB (left) and robotic VSG (right). We use all four robotic arms for the robotic RGB and only use three arms for the robotic VSG. After all the trocars are placed, the circulator will drive the robot, positioning it over the patient’s head (Figure 4). During this step, the sterile team members and the circulator must be aware of the sterile robotic arms in relation to unsterile equipment. Also, all staff must monitor the robot when it nears the patient’s head, though the head wrap will offer a margin between skull and robot as another element of protection. Once the robot is positioned, robotic arms will be secured to the robotic trocars and instruments will be inserted. The robotic fenestrated bipolar forceps, Maryland bipolar forceps, Cadiere forceps, Cautery scissors, Cautery hook and double fenestrated forceps were used in robotic RYGB. And only fenestrated bipolar forceps, Cautery hook and double fenestrated forceps were used in robotic VSG.
The standard procedure of both robotic RYGB and VSG have been described in many studies (8-11). Figure 5 shows anatomy pictures of gastric bypass (left) and sleeve gastrectomy (right) after they were done. After the procedures, the intralumen anastomosis is insufflated using a gastroscope (under extralumen irrigation) to verify the absence of leakage. All the robotic instruments were removed and robot was moved away from patients. Then all the ports are removed and the skin incisions are sutured closed.
Discussion
The operative cares for robotic procedures are the same as for traditional laparoscopic procedures, and patient education is a key component to recovery for both styles. For intra-operative patient care, the robotic surgery requires more staff than most other surgical procedures, and, in hopes of keeping a safe and orderly work place, the operating room requires highly trained staff to prepare the surgical robot and ensure its appropriate maintenance (12,13). In many institutions, specialized teams work with robotic surgeons on a regular basis (13) and many hospitals are requiring that the nurse coordinator of any given robotics program become as specialized as the doctors doing the actual surgery (13). The expertise of a seasoned coordinating nurse, circulating nurses, surgical technologists, and surgical assistants puts the surgeon more at ease and provides for better, consistent results for the patients we serve.
The robot would generally be prepared before the patient arrives to the operating room. The bedside staff should understand how to install instruments and remove them from the robotic arm ports. Perioperative nurses must be proficient in positioning the robot components in relation to the patient and all other equipment in the room. The nurse sustains vigilance regarding all aspects of the procedure. Prior to admitting the patient into the operating room suite, the nurse should make sure that the staff has any necessary information about the patient. The nurse should also watch the surgeon at the console, the display monitor showing the surgeon’s activities inside the abdomen, and the sterile team members and equipment that they must utilize. This is to insure that all aspects of the surgery are progressing smoothly and to anticipate needs for the remainder of the procedure, retrieving any items that may be required by other team members. The nurse also communicates with the anesthesiologist to assure that the vital signs are satisfactory, that the patient is achieving adequate respiratory excursion (14) and that intravenous lines are running well; this is a task that begins during the positioning stage until the patient leaves the operating room.
Most importantly, though, the nurse will keep careful watch over the patient which includes: (I) padding pressure points at the beginning of the case and rechecking those areas during the procedure; (II) observation of the robotic arms throughout the procedure to make sure the patient will not be struck with the arm movements; (III) observing the patient’s position throughout the case to insure that nothing would cause the patient to shift on the table.
Conclusions
The robotic-assisted bariatric surgery is safe and feasible over open approach even more time in the operating. After understanding the procedures, room setup, intra-op patient’s care and teamwork, the robotic approach of bariatric surgery should be in the surgeon’s skillset.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.07.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study did not require any approval by institutional ethical committee or informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 2012;307:483-90. [Crossref] [PubMed]
- Crawford AG, Cote C, Couto J, et al. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE Centricity Electronic Medical Record database. Popul Health Manag 2010;13:151-61. [Crossref] [PubMed]
- Wittgrove AC, Clark GW. Laparoscopic gastric bypass: a five-year prospective study of 500 patients followed from 3–60 months. Obes Surg 1999;9:123-44. [PubMed]
- Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg 2000;10:514-23. [Crossref] [PubMed]
- Deng JY, Lourié DJ. 100 100 robotic-assisted laparoscopic gastric bypasses at a community hospital. Am Surg 2008;74:1022-5. [PubMed]
- Horgan S, Vanuno D. Robots in laparoscopic surgery. J Laparoendosc Adv Surg Tech A 2001;11:415-9. [Crossref] [PubMed]
- Tabor W. On the cutting edge of robotic surgery. Nursing 2007;37:48-50. [Crossref] [PubMed]
- Ayloo SM, Addeo P, Buchs NC, et al. Robot-assisted versus laparoscopic Roux-en-Y gastric bypass: is there a difference in outcomes? World J Surg 2011;35:637-42. [Crossref] [PubMed]
- Jacobsen G, Berger R, Horgan S. The role of robotic surgery in morbid obesity. J Laparoendosc Adv Surg Tech A 2003;13:279-83. [Crossref] [PubMed]
- Ijah RF, Bhatia P, Kaltan S, et al. Sleeve Gastrectomy for Morbid Obesity: Robotic vs Standard Laparoscopic Sleeve Gastrectomy Methods. World J Lap Surg 2014;7:1-6.
- Ayloo S, Buchs NC, Addeo P, et al. Robot-assisted sleeve gastrectomy for super-morbidly obese patients. J Laparoendosc Adv Surg Tech A 2011;21:295-9. [Crossref] [PubMed]
- Mohr CJ, Nadzam GS, Alami RS, et al. Totally robotic laparoscopic Roux-en-Y Gastric bypass: results from 75 patients. Obes Surg 2006;16:690-6. [Crossref] [PubMed]
- Francis P. Evolution of robotics in surgery and implementing a perioperative robotics nurse specialist role. AORN J 2006;83:630-42, 644-6, 649-50 passim. [Crossref] [PubMed]
- Neil JA. Perioperative nursing care of the patient undergoing bariatric revision surgery. AORN J 2013;97:210-26. [Crossref] [PubMed]
Cite this article as: Levine JW, Feng Z, Feng DP, Melvin WV. Perioperative patient care involved with robotic-assisted bariatric surgery. Ann Laparosc Endosc Surg 2017;2:136.