How I do it: technical tips of laparoscopic surgery for advanced gastric cancer
Laparoscopic distal gastrectomy with radical lymphadenectomy for early gastric cancer is technically established, and it has penetrated clinical scene worldwide, especially in East Asian countries, where the incidences of stomach cancer are higher than the rest of the world (1-3). Meanwhile, in terms of expanding its indication to more advanced gastric cancer, there are still a lot of arguments in both technical and oncological viewpoints. In East Asian countries, several large-scaled prospective randomized studies have been conducted to prove non-inferiority of laparoscopic surgery to open surgery, and presently patients’ accruals of these trials have been completed (4-6). Now we are watching over their upcoming long-term outcomes for reaching final conclusion. Technically, without doubt accurate complete D2 dissection is necessary for advanced gastric cancer, and additionally in whatever situation surgeons should follow oncological principles, not to cause cancer cell spillage or local residue due to inadequate dissection during the surgery. In fact, in large tumors or in cases with actual nodal metastases, surgical maneuvers tend to be even more difficult than usual prophylactic lymphadenectomy in early cancer. In this article, we focus on our technical tips of performing laparoscopic surgery for advanced gastric cancer aiming safe procedures in technical as well as oncological aspects.
Indication of surgery
Safety and feasibility of laparoscopic distal gastrectomy for cStage II/III gastric cancer has been demonstrated by several prospective large-scaled studies, which compared with open surgery (4-6). On the other hand, oncological validity in terms of long-term outcomes has not been well shown. In our institution, sufficient informed consent is offered to all of the patients with explaining currently available scientific evidences, and if it is obtained, laparoscopic surgery is applied. Huge tumors larger than 8 cm, type 4 tumors, or tumors with gross lymph nodes metastases adjacent to the major vessels are out of the indication in our institution.
Basic set-up, necessary instruments and port placement
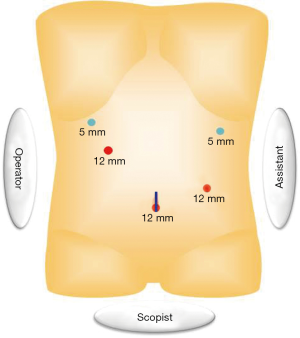
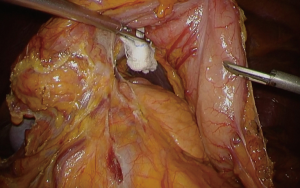
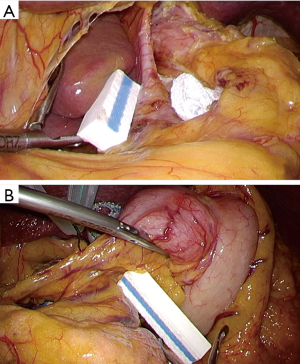
When a surgical team has enough experiences of laparoscopic surgery with prophylactic lymphadenectomy for early stage gastric cancer, basic concept of surgical approach can be extrapolated. Our standard port placement is shown as Figure 1, which is almost same as that for early cancer, but the right lower port is placed a little closer to median to facilitate approach to supra-pancreatic region by energy devices. A camera port is placed above or below the umbilicus according to the patient’s habitus; and as for optics we routinely use a 12-mm flexible high-definition endoscope (VISERA ELITETM, Olympus, Tokyo, Japan) to provide better visualization around the supra-pancreatic area or the splenic hilum, where views from upside obtained by articulation function of the flexible endoscope up to 90 degrees is effective. In most of operative steps, the operator stands at the right of patient except dissection of No.6 nodal station. The assistant surgeon stands at the opposite side, and the camera assistant between patient’s legs. In advanced cancer, surgical situation may be tougher owing to tumor’s progression. In general, tissue around the lesion is likely to be wet or sometimes firm due to inflammation related with tumor infiltration. Especially, excessive lymphatic discharge tends to hamper placid dissection. During the surgery, we place a couple of water absorptive surgical sponges (KAIMENTM, Kawamoto Corporation, Osaka, Japan) in the operative fields, exactly besides the right diaphragmatic crus and between the stomach and the pancreas body, in which space discharge are easily pooled (Figure 2). By using this, dry operative fields can be kept. Suction device is also frequently used to suck superfluous discharge. Specialized gauze for endoscopic surgery (TROXTM, Oosaki Medical, Nagoya, Japan) is essential to be used for gentle retraction of the organs (Figure 3).
Omentectomy
In T3-4 tumors in surgical findings, omentectomy is recommended in the current Japanese guidelines (7). Survival impact of bursectomy, which excises the anterior leaf of the transverse mesocolon as well as capsule of the pancreas aiming complete removal of micrometastasis on the neighboring peritoneum, has been denied by JCOG 1001 randomized trial, which compared omentectomy versus bursectomy (8). Nowadays bursectomy is not performed as a routine procedure anymore in Japan. Furthermore, some researchers have suggested the doubt even in potential effectiveness of omentectomy, because there has been no persuasive scientific evidence to verify its survival impact. Regarding this issue, further investigations with well-designed prospective studies are required. In this context, presently omentectomy is regarded as a standard procedure for T3–4 tumors.
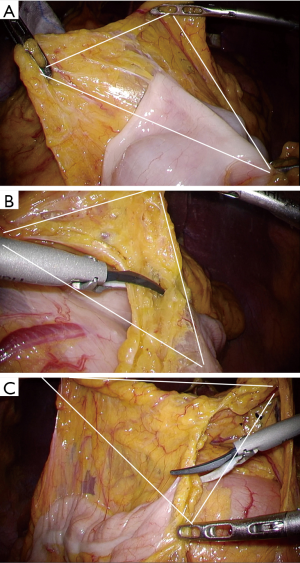
Technically, in laparoscopic surgery, wide traction of the transverse mesocolon is not so as easy as in open surgery. For overcoming this limitation, triangulation to make tension to the attachment between the omentum and the transverse colon in cooperation with assistance is essential in laparoscopic surgery (Figure 4). When the posterior wall of the stomach is recognized by sight, we can confirm entering the omental bursa. Once the space of the omental bursa is identified, another triangulation should be formed to make tension to the attachment between the omentum and the transverse colon. On the right side of patients, physiological adhesion of the anatomical structure is more complex, so that the correct plane in front of the transverse mesocolon should be carefully kept. During the procedures of omentectomy, head-up tilt of the operative table is necessary to extent the transverse colon mesentery using the traction by the gravity.
Management of metastatic lymph nodes
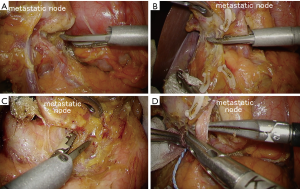
As mentioned, laparoscopic surgery techniques of prophylactic D2 lymph node dissection have been almost established. However, when encountering swollen lymph nodes with actual metastasis, technical difficulty will remarkably increases. Surgeons should pay the utmost attention not to cause destruction of the nodal capsule to maintain safe excisional margin (Figure 5). Careless manipulation with forceps will cause damage of the nodal capsule, which will lead to cancer cell spillage. In order to avoid such adverse events, surgeons should not directly grasp swollen nodes. We sometimes use forceps with closing this jaw, and just push up structures including metastatic nodes. Once, the proper dissection plane is exposed, it is important to keep this plane without any hemorrhage. Nevertheless, in extensive metastatic cases, finding the dissection plane is difficult between tumor and the preserving organs. Laparoscopic surgery lacks tactile feedbacks, thus in such cases we use a fine dissector, which is useful to find the dissection space or make a decision regarding its resectability.
Management of large tumor or tumors with serosal invasion
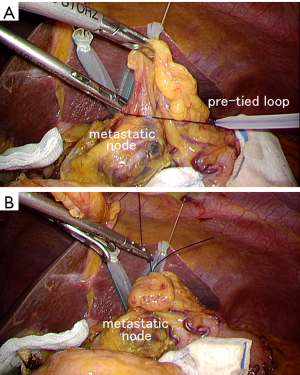
In laparoscopic surgery, surgeons cannot use their hands for retraction. Thus, in large tumors with serosal invasion, gentle manipulation without surgeon’s hand is really crucial to prevent cancer cell spillage in the abdominal cavity. Direct grasping of gastric wall using forceps should be absolutely avoided. Retraction using gauze can be effective for atraumatic handling as an alternative solution. We sometimes utilize pre-tied loop to ligate perigastric fat tissue to be lifted up through the abdominal wall (Figure 6). Tilt function of the operative table should be considerably used, in which the gravity can be utilized for retraction. Head-up or left side-up tilt is effective to obtain sufficient visibility around the splenic hilum. Nonetheless, we believe gross tumors larger than 8 cm are not appropriate candidates for laparoscopic surgery due to oncological safety.
Trouble-shoot for intraoperative hemorrhage
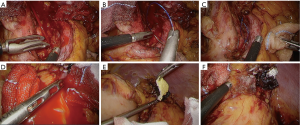
In operation for advanced gastric cancer, we have more probability to encounter intraoperative troubles, such as hemorrhage from the major vascularity. When major hemorrhage is recognized during the surgery, surgeons should attempt to achieve primary hemostasis by gauze compression or by grasping a bleeding point directly by forceps. Once tentative hemostasis is obtained, blood in the operative fields should be sucked or swept to make the relevant anatomy clear, eventually the bleeding point may be correctly understood. If the target vessel is separated from other structures, a detachable vascular clip is useful for controlling hemorrhage or subsequent hemostatic procedures. Regarding means to complete hemostasis, there are various options, such as clips, suturing, pre-tied suture loop or tissue sealing sheet, and surgeons should choose a proper one considering each situation. Suturing is one of the most reliable ways to control bleeding; however, for accurate procedures enough proficiency is required (Figure 7A,B,C), particularly for that from the major vein. Currently, tissue sealing sheet are available, which is effective for hemostasis on the vein. We always prepare one of that (TachoSilTM, CSL Behring, King of Prussia, PA, USA) for emergent situation (Figure 7D,E,F).
Postoperative managements
We usually put 19-Fr closed-type drainage tube with continuous aspiration along the supra-pancreatic rim. Amylase levels of the discharge in the tube are measured on 1st and 3rd postoperative days. When there is no abnormal elevation of the amylase levels or cloudiness in discharge, the tube is removed on the 4th postoperative day. Drinking water is started on 1st postoperative day, and soft meal is on 3rd postoperative day. When there is no complication, patients are allowed to be discharged on 7th postoperative day.
Acknowledgments
T. Kinoshita received honorarium from Olympus, Johnson & Johnson, Medtronic, and Intuitive Surgical.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.10.08). TK serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from May 2016 to Apr 2018. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Honda M, Hiki N, Kinoshita T, et al. Long-term outcomes of laparoscopic versus open surgery for clinical stage i gastric cancer: the LOC-1 Study. Ann Surg 2016;264:214-22. [Crossref] [PubMed]
- Katai H, Mizusawa J, Katayama H, et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer 2017;20:699-708. [Crossref] [PubMed]
- Kim W, Kim HH, Han SU, et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage i gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg 2016;263:28-35. [Crossref] [PubMed]
- Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol 2016;34:1350-7. [Crossref] [PubMed]
- Inaki N, Etoh T, Ohyama T, et al. A multi-institutional, prospective, phase ii feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg 2015;39:2734-41. [Crossref] [PubMed]
- Lee HJ, Hyung WJ, Yang HK, et al. Morbidity of laparoscopic distal gastrectomy with D2 lymphadenectomy compared with open distal gastrectomy for locally advanced gastric cancer: Short term outcomes from multicenter randomized controlled trial (KLASS-02). J Clin Oncol 2016;34. abstract 4062.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (Ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- Terashima M, Doki Y, Kurokawa Y, et al. Primary results of a phase III trial to evaluate bursectomy for patients with subserosal/serosal gastric cancer (JCOG1001). J Clin Oncol 2017;35. abstract 5.
Cite this article as: Kinoshita T, Tokunaga M, Kaito A. How I do it: technical tips of laparoscopic surgery for advanced gastric cancer. Ann Laparosc Endosc Surg 2017;2:162.