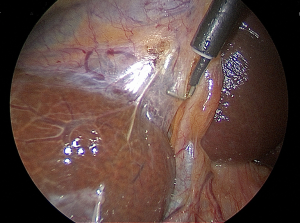
Critical view of safety—its feasibility and efficacy in preventing bile duct injuries
Introduction
In the late 1980’s, surgeons introduced laparoscopic cholecystectomy (LC) as an alternative to conventional cholecystectomy. This procedure along with the minimally invasive surgery (MIS) revolution which soon followed was one of the greatest advancements for patients in the history of medicine and surgery. LC quickly became mainstream and the gold standard method of management of cholelithiasis (1). Because an entire generation of surgeons needed to undergo training in this new technique or risk loss of referrals, short 2- to 3-day courses that consisted of didactic lectures and workshop/training on models and in animal labs were developed to accommodate the need for surgeon retooling. Surgeons were unaware initially of the complications of this newer technology, although early reports suggested an increase in the rate of bile duct injury (BDI) (2). Considering the large numbers of LC’s being performed all over the world, the implications in terms of patient morbidity and mortality, quality of life, and costs to the health care system were considerable (3-9).
As the problem of BDI became more apparent, the surgical community undertook different strategies and innovations to overcome the challenges of this newer technique. This brought into focus reviewing and understanding the causes and mechanism of these injuries in context to variable and unpredictable anatomy and other factors in dealing with LC (10-13). Despite advances in knowledge, technique and technology, the incidence of BDI remains higher than that which occurred historically in the era of open cholecystectomy (14). Over the last 20 years, various strategies have been proposed to find a solution to the underlying causes of BDI such as misidentification of ductal anatomy, human psychological factors, and improving technique and utilizing technology. Several studies have examined the role of utilizing different means of intraoperative imaging such as cholangiography and laparoscopic ultrasound to assist the surgeon to eliminate the error traps of misidentification of ductal structures (15-17). However, while these modifications and strategies are useful and applicable in many patients, the overall results have not been absolute, and surgeons have not often embraced routine intraoperative imaging and so room for improvement remains.
One major risk factor for biliary injury is variability in anatomy. According to the latest literature by Singh et al. (10), variable anatomic structures include subvesical ducts, accessory ducts, a right hepatic duct (RHD) with a low entry into the common hepatic duct (CHD) (or into which the cystic duct empties), a cystic artery that courses lateral to the cystic duct, and other variations. In addition, in both acute and chronic inflammatory conditions, the relationships of the cystic duct, RHD and common bile duct (CBD) may be distorted and less predictable. This means the extent of dissection must account for this variability to avoid inadvertent injuries (10,12,17).
Hence, the best strategy for the surgeon is to perform a safe dissection in which the relevant structures are identified irrespective of their normal or abnormal arrangements. Analyzing the pattern of BDIs has shown that misidentification of CBD or hepatic duct as the cystic duct is the most common mechanism of injury. Therefore, an approach was needed to reliably ensure that no other structures would be mistaken for the cystic duct and artery and inadvertently clipped or divided (11,18).
In 1995, Strasberg et al. introduced a method of ductal identification termed the “critical view of safety” (CVS) (19). The CVS is not a dissection technique but rather a method of identification based on the concept of thorough dissection and delineation of all the structures in the hepatocystic triangle. The purpose of this review is to highlight the important principles of achieving the CVS and to analyze outcomes and challenges of this approach along with its limitations.
What is the CVS?
The principle of the CVS is a based on an extended dissection and delineation of all structures in the hepatocytic triangle as a method of identifying cystic duct and the artery conclusively and avoiding misidentification injuries. The CVS was conceptualized after an analytical review of bile duct injuries associated with LC (3). The CVS was also based on experience from open cholecystectomy in which the cystic duct and artery were isolated and then the gallbladder was completely taken off the liver bed which left only two structures attached to it—the cystic duct and artery—with no potential for misinterpretation of the anatomy (19). Althsough the CVS is the most stringent method available for ductal identification today, there has been an incomplete understanding of the requirements and, therefore, it has not been uniformly carried out (19). This review analyzes the available data on CVS with two aims in mind:
- Why surgeons have not adopted this method universally? Is it a sufficient method to prevent bile duct injuries or there is a need to complement it by some other method?
- The limitations of its application and effectiveness.
The CVS has three requirements as shown in Figure 1. First, the hepatocystic triangle is cleared of all fat and fibrous tissues. Second, the lower one-third of the gallbladder is dissected off the liver to expose that portion of the cystic plate. The cystic plate by definition is the white fibrous tissue where the gallbladder is attached to the liver. The third component of the CVS is that two and only two structures are seen to enter the gallbladder (19). All three criteria must be fulfilled in order to claim that the CVS has been achieved. The basis of this extended dissection requires that the anatomy be visualized through a 360-degree viewpoint. The prerequisite of achieving the CVS is a more rigorous dissection compared to other methods which is in part why it is valuable, and which can alert one to the presence of variable or unpredictable anatomy or a difficult dissection in the setting of acute or chronic inflammation (10,19).
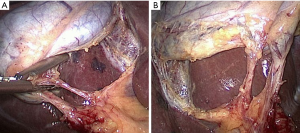
Key points in dissection
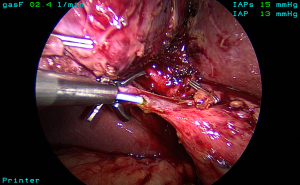
The standard technique of LC is carried out with four ports and using a 30-degree laparoscope. The gallbladder is grasped at the fundus and retracted towards the right shoulder. The infundibulum is retracted inferolaterally to open up the hepatocystic triangle (Figure 2). Cephalad traction should be avoided as it may tent the CBD and align it with the neck of the gallbladder increasing the risk of misidentification. A combination of blunt and electrosurgical dissection may be used to open the peritoneum at the gallbladder neck and dissect the hepatocystic triangle working back and forth from the anterior and dorsal views (Figure 3). Energy should be used in brief 2–3-second intervals to limit any thermal spread beyond the area of dissection. Although beyond the scope of this article, it should be emphasized that the principles of dissection remain the same for other approaches such as single incision (20) or robotic techniques.
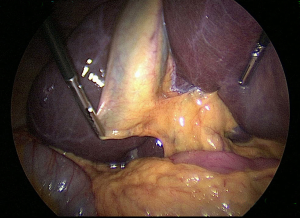
Application of CVS methods
Assessment of CVS
A new concept when reaching the CVS is for the operating team to pause and take a timeout to verify that all three components of CVS are achieved (21). The time out serves simply as a stop point to ensure that the anatomy is verified before proceeding with clipping or cutting any ductal structures. One may also verify the anatomy by performing intraoperative cholangiography (IOC) at this stage in the operation (see section below). Attainment of the CVS may be documented by video recordings (see video clip, Figure 4) and/or photographs. Sanford et al. showed that photographs that delineated the CVS from both the anterior and posterior views (doublet photo, see Figure 1) could be used to reliably confirm that the CVS has been reached (23). They used a 6-point scale to assess achievement of the CVS that awarded up to two points for each component reached. Other groups have suggested that videos are superior to photographs when assessing the CVS (24) and that operative notes are less reliable than photographs or videos (25,26)). In one study that analyzed lap cholecystectomy videotapes of ten surgeons (27), only 20% of surgeons achieved a CVS score of 6, whereas the remaining eight had a mean score of 1.75 and two surgeons had a score of zero. Following a coaching intervention for five of the surgeons with low initial scores, the mean CVS scores rose to 3.75. An example of an incomplete CVS is shown in Figure 5.

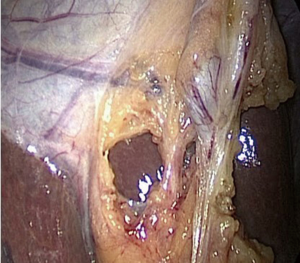
Several groups have shown that operative notes may state the CVS was obtained when in fact on video review it was not. Nijssen et al. (28) in 2016, however, found that, even after an educational intervention that consisted of a lecture, handout, and teaching video, surgeons failed to consistently achieve the CVS. In their study, agreement between operative notes and video analysis was analyzed in 229 videos of 369 patients who underwent LC. According to the operative notes, CVS was reached in 92% cases but on video review, the CVS was achieved in 76% of cases. Furthermore, the percentage of cases in which CVS was reached increased but not significantly between the pre-intervention, first post-intervention and second post-intervention groups (from 68.5% to 73.3% to 82%). Consequently, as a possible strategy, we suggest that surgeons dictate in the operative note that all three elements of the critical view were attained and to consider photographic or video documentation as well. A summary of the results of CVS assessment is given in Table 1.
Table 1
| Authors & years | No. of patients | Methods | CVS results | Conclusions |
|---|---|---|---|---|
| Deal et al., 2017 (29) | 160 | Video | Criteria CVS ≥5 achieved in 12.5% | High degree of correlation between crowd worker and faculty experts in assessment of CVS |
| Deal et al., 2017 (30) | NA | Developed a tutorial to teach CVS. Experts, lay persons and crowd workers to get inputs | NA | Multimedia educational tutorial can be developed using a step-wise interactive approach to teach learners, how to assess CVS and LC |
| Nijssen et al., 2016 (28) | 229 | Video | CVS achieved in 69% | Even after teaching interventions complication rate and rate of achieving CVS was not improved significantly |
| Stefanidis et al., 2016 (27) | 10 | Video | CVS achieved in 20% | CVS criteria not routinely used by majority of participating surgeons |
| Nijssen et al., 2015 (31) | 1,108 | Video | 80%, according to operative notes; |
Evaluation of surgical videos of LC cases are important |
| Sanford et al., 2014 (23) | 28 | Doublet photography | CVS achieved in 78% cases | With training and adherence to straightforward photographic techniques intraoperative, doublet photography can record CVS accurately |
| Plaisier et al., 2001 (26) | 50 | Comparison of operation notes, video images and photo prints | CVS achieved in 78% cases | Video proved superior to photoprints regarding quality |
| Photoprints practically and logistically more easily produced than videos | ||||
| In good quality photos, CVS recorded more conclusively than video |
NA, not applicable; CVS, critical view of safety; LC, laparoscopic cholecystectomy.
What is the evidence that the CVS works?
A prospective, randomized trial to demonstrate that the critical view of safety reduces biliary injuries would not be possible because it would require over 10,000 patients due to the low overall rate of biliary injury. However, there is secondary evidence that the CVS method of structure identification is effective. The first is that there are case series in which large numbers of patients were studied in whom the CVS was used without any biliary injuries due to misidentification (32,33). The expected rate of biliary injury in these cases would have been at the 0.2–0.4% level which would have resulted in approximately 15–20 such injuries. The other primary evidence is that studies in which the mechanism of BDI was analyzed have generally not had the CVS described as the method of identification used (31,34). The CVS also works because it replicates the proven method of ductal identification in open surgery, and it is also demanding. When one has difficulty getting to the critical view, this should indicate the potential for danger and consideration should be given to altering the approach.
Evidence also exists that the critical view of safety as described by Strasberg is misunderstood and not widely applied. Nijssen and colleagues in a study of 1,108 consecutive laparoscopic cholecystectomies in the Netherlands found an overall BDI rate of 1.7%. Of these 0.6% were major BDIs (31). In this analysis, 65 surgical videos of cases with complications were reviewed in detail. The operative notes indicated that the CVS was achieved in 80%, but on video review the authors found that the CVS was actually achieved in only 10.8% of cases. Furthermore, agreement between what was stated in the operative notes and the video occurred in only 18.7% of patients. Most importantly, the CVS was not reached in any of the patients with biliary injuries.
A survey study of 374 surgeons found that the CVS could not be correctly identified in descriptive terms by 75% of the surgeons and that 21% could not identify it visually (35). Rather, the infundibular method was practiced by 56% of surgeons and the CVS method by only 27%. Only 16% of surgeons used cholangiography routinely. Recently, Deal and associates carried out an evaluation of existing on line surgical videos using a combination of faculty experts and crowd source worker assessment (30). A total of 160 blinded surgical videos comprising the segment of the dissection just prior to clipping and cutting any structures in the hepatocystic triangle were analyzed. Using the 1–6 doublet scale where 6 meets all criteria of the critical view both in the anterior and posterior view, the average score was only 3.6 as rated by crowd workers and 3.8 by faculty experts. Of note is that only 12.5% of the videos rated achieved a CVS score of ≥5, which indicates most videos were inadequate in their demonstration of the CVS. These findings suggest the need for further education regarding the CVS and how to appropriately demonstrate that the CVS has been obtained operatively (29). A summary of cases series in which the CVS was utilized with the rate of CVS achievement and BDI rate is shown in Table 2 (37-39).
Table 2
| Authors | Case data | CVS achieved | BDI rate |
|---|---|---|---|
| Avgerinos et al., 2009 (33) | 1,000 patients | 95.4% | 0% |
| Yegiyants and Collins 2008 (32) | 3,042 retrospective | NS | 1 major BDI (0.03%) |
| Sanjay et al., 2010 (36) | 447 [40% acute cholecystitis (IOC selective)] | 87% | NS |
| Tsalis et al., 2015 (37) | 929 retrospective, 873 available, IOC selective | 95.8% | 0% |
| Heisterman 2006 (38) | 100 patients | 97% | 1.6% |
| Rawlings et al., 2010 (20) | 54 patients, SILS; |
64%, 3 criteria; |
0% |
| Kaya et al., 2017 (39) | 120 patients, no IOC | 100% | 0% |
| Nijssen et al., 2015 (31) | 171 videos reviewed | 80%, per operative notes; |
0.6%, types B, D, E; |
NS, not stated; SILS, single incision lap chole; BDI, bile duct injury; IOC, intraoperative cholangiography; CVS, critical view of safety.
What to do when the CVS cannot be obtained?
IOC and other imaging adjuncts
In the setting of a difficult gallbladder in which the CVS cannot be obtained, this should first alert the surgeon to the potential for danger in persistence with the dissection. Several strategies are available under these circumstances. One is to do a cholangiogram or other means of intraoperative biliary imaging. It is important to note that IOC should be considered an essential skill for surgeons who perform LC. In addition, when interpreted properly, it may prevent a higher level of injury by avoiding resection of the CBD and also result in more time repair. However, it may not always detect aberrant ducts and, therefore, should be used as an adjunct to and not a substitute for careful dissection and utilization of the critical view of safety.
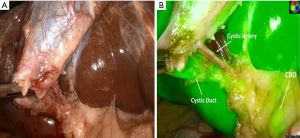
Cholangiography is typically done through the cystic duct (Figure 6) but can be carried out through the neck of the gallbladder if necessary. Several studies have examined the influence of IOC on common BDI rates. In one analysis of 40 reports in the literature, the incidence of biliary injury was 0.21% for routine cholangiography compared to 0.43% in those with selective use (40). When a BDI did occur, the diagnosis of it was made at the time of cholecystectomy in 87% of routine IOC cases vs. 44.5% with selective IOC. The conclusions of this study were that IOC reduced the incidence of severe bile duct injuries and increased detection at operation. Flum and colleagues (41) in 2003 carried out a Medicare database analysis of 1,578,361 lap cholecystectomies. CBD injuries occurred overall in 7,911 (0.5%). The rate of injury in patients who did not undergo IOC was 0.58% compared to 0.39% in those who had a cholangiogram. This translated to an increased relative risk of injury of 1.79 for patients who did not have an IOC. In another study of over 150,000 cholecystectomies in Sweden, IOC was found to reduce the CBD injury by 34% (42). Recently, the Swedish Gallriks study group reported on 51,404 cholecystectomies performed from 2005–2010 (43). In this series, there were 747 BDIs for a rate of 1.5%. They found that the intent to use IOC led to earlier detection of injury and reduced the risk of death by 69%. However, controversy on this issue remains. Sheffield and colleagues examined over 92,000 Medicare claims from Texas Between 2000–2009 (44). The rate of usage of IOC was 40.4% in that study. When they controlled for confounding variables using instrument variable analysis, there was found to be no significant association between use of IOC and duct injury.
Some groups have utilized laparoscopic ultrasound imaging for anatomic verification but most surgeons are less familiar with its use and interpretation. Recently, infrared cholangiography with indocyanine green has been advocated as an alternative means for imaging the biliary tree during cholecystectomy (45,46). The advantage of this approach is that it allows continuous biliary mapping in which one can alternate back and forth between white light and fluorescence imaging to visualize the bile duct and/or cystic duct (Figure 7). The limitations of this technology are that it has not been widely studied nor has it been evaluated sufficiently under many conditions of difficult cholecystectomy such as acute cholecystitis and in obese patients.
Conversion to open cholecystectomy
A second option in the difficult gallbladder when the CVS cannot be obtained is to convert to an open cholecystectomy. Traditionally, this has been the preferred approach if progress is not being made and the laparoscopic dissection is too difficult (7). However, many surgeons today have trained in an era where they received limited experience with open cholecystectomy and, therefore, may not have the appropriate experience to manage a difficult gallbladder in an open fashion. In addition, conversion to an open operation does not necessarily protect against biliary injury. In one study of 1,089 patients who were undergoing LC for acute cholecystitis in Belgium, the biliary complication rate was 13.7% in 116 patients who were converted from laparoscopic to open procedures (47). There were seven major bile duct injuries in the converted group, 3 of which occurred after conversion.
Get help from a colleague
A third option is to get help from an experienced surgical colleague. This approach is likely underutilized but can be helpful in terms of providing a different prospective and viewpoint of the operative field. This is also one of the six steps in the SAGES safe cholecystectomy program as will be described below. To this point, in a recent study using an insurance claims database (48), surgeons who were less experienced were found to be more likely to have incurred a BDI than those who had been in practice for more than 5 years.
Exit strategies
Finally, one could utilize a bail out strategy in these cases. One option would be to terminate the laparoscopic procedure and refer the patient to a tertiary center with hepato-pancreatic-biliary (HPB) and/or advanced laparoscopic surgery expertise. Alternatively, a cholecystostomy tube could be inserted into the gallbladder laparoscopically which should alleviate the acute condition, and then the patient could undergo cholecystectomy 2–3 months later once the inflammatory process had settled. Finally, if the hepatocystic triangle can be reached but not safely dissected, a laparoscopic subtotal cholecystectomy should be considered (49,50).
Our group has described two subtypes of subtotal cholecystectomy, fenestrating and reconstituting (50). In the reconstituting approach, the neck of the gallbladder is sutured or stapled off whereas in the fenestrating procedure, the gallbladder neck is left open and the cystic duct either sutured from within the lumen of the neck of the gallbladder or left open. One generally leaves about 2–3 cm of the gallbladder neck to protect the area of the hepatocystic triangle and porta hepatis (Figure 8). Impacted stones should be removed and a cholangiogram can be done through the cystic duct if it can be identified. The remainder of the gallbladder is removed although the back wall may be left in place. The gallbladder bed should be coagulated and the gallbladder fossa drained. It should be noted that the term partial cholecystectomy is imprecise and, in our opinion, should be abandoned in favor of the two types of subtotal resection.
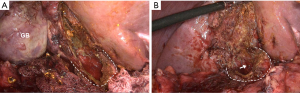
van Dijk and colleagues recently reported outcomes of laparoscopic subtotal cholecystectomy in 191 patients in four teaching hospitals in the Netherlands (51). Of these, 53% had a fenestrating subtotal cholecystectomy and 38% a reconstituting subtotal cholecystectomy. Postoperative bile leaks occurred more commonly after the fenestrating procedure than reconstituting (18% vs. 7%). At a median follow-up interval of 6 years, recurrence of biliary symptoms was lower after the fenestrating than reconstituting approach (9% vs. 18%). Of note is that there was only one BDI in this series and that was in the reconstituting subtotal cholecystectomy group. These data suggest that laparoscopic subtotal cholecystectomy is an acceptable alternative in high risk patients, but that complications and recurrent symptoms will occur in a significant percentage of patients, and so this approach should be used judiciously.
Factors influencing the outcome of CVS in LC
Teaching intervention
The influence of teaching intervention in reaching the CVS has been studied but data analysis show that although there is an encouraging influence, additional strategies are needed. Nijssen et al. (28) in a study of cases in which the CVS was not reached, concluded that it would have been possible to reach the CVS in many of these cases if just the lower one-third of the gallbladder had been dissected from the liver bed. However, the two-structure view does tend to improve after teaching interventions. Also, the 360-degree view of the cystic duct entering the gallbladder improved significantly. Personal intervention was also more effective than group intervention.
Human psychological factors
Cognitive psychology has given rise to a subspecialty dealing with the science of human error which has recently been applied to analyze complications of BDIs. Way et al. (52) analyzed BDIs that occurred from laparoscopic cholecystectomies and found that the primary cause of error in 97% of cases was one of visual perception/illusion rather than surgical skill and knowledge. The existence of videotapes and operating notes from cases where the injuries were not diagnosed during the operation allowed the events as seen by the surgeon to be compared with reality as recorded on the videotapes, post injury X-rays, and the findings during reparative operations. In each case, it was judged that the surgeon carried out the procedure with adequate skill, blood loss was minimal, and the tissue planes were dissected cleanly but in retrospect, incompletely. The cause of these injuries in other words was not at the action end of the sequence (i.e., skill). The phenomenon of misperception in such cases is termed cognitive fixation.
In class 1, class 3 and some class 4 injuries, misidentifying the common duct (or RHD) for the cystic duct was the mistake observed, which resulted in partial injury or transection of the misidentified duct. In class 2 and some class 4 injuries, the mistake consisted of performing the dissection in the hepatocystic triangle unintentionally too close to the border of the CHD or RHD. The underlying nature of mistake in either case was the misperception of the operating surgeon.
In cases of class 3 injuries where the CBD was completely transected, it was found that the anatomic relationship between the CBD and the gallbladder mimicked the surgeon’s mental model of the relationship between the cystic duct and the gallbladder. This illusion was compelling and, thus, was accepted by the surgeon as reality. Class 3 injuries of the bile duct were rare in the era of open cholecystectomy. This finding suggests that the laparoscopic environment may predispose to misperception whether due to loss of haptic information, loss of depth perception, or limitations in perspective (position of laparoscope is fixed). These factors may be mitigated by discussion among the operating team members (see intraoperative time out above) or input from other colleagues.
More recently, factors that surgeons felt impacted the occurrence bile duct injuries during LC were analyzed in a survey study from one major health care system (53). Overall there were 84 BDI cases (0.1% rate), the majority of which were cystic duct leaks, but with a trend toward more severe, proximal injuries over time. Of the 56 surgeons who responded to the survey, factors cited that predisposed to BDIs were lack of surgeon experience (70.9%) and a higher threshold for converting to open cholecystectomy (43.6%). This higher threshold for conversion as stated meant that surgeons were less likely to convert due to less experience with open cholecystectomy and a “fear” of conversions, thus continuing with the laparoscopic technique possibly beyond safe limits. Also, 13% of the surgeons felt that impatience or boldness contributed to injuries with too much importance placed on speed. Of surgeons who experienced a BDI, 40%stated they had considered conversion to open cholecystectomy at some point during the operation, but did not do so because of confidence in their laparoscopic skills. Of note is that 48.3% thought earlier conversion to an open approach would have prevented a BDI. None of the surgeons in this study employed cholangiography routinely.
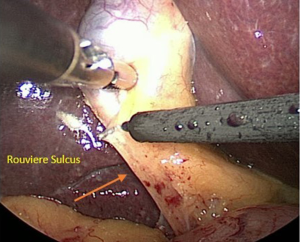
When a surgeon performs cholecystectomy, the subconscious mind seeks a design to match the mental model of the biliary structure stored in the surgeon’s memory. The tissues that he/she inspects may be hidden by connective tissue, inflammation or blood. As a result, the decision regarding the dissection is made from a pattern consisting of signals and noise. The surgeon should also be alert to other anatomic clues that indicate the location of critical structures. For example, Rouviere’s sulcus is a cleft in the liver which demarcates the plane of the CBD and porta hepatis (Figure 9). It is important to maintain the dissection anterior to this plane. We would argue that the CVS method diminishes the misperception factor that leads to BDI by allowing the surgeon to view the anatomical structures from all angles and to eliminate the possibility of a duct being divided that does not transit only to the gallbladder.
Why the CVS is not utilized routinely in LC?
The incidence of biliary injuries has not declined significantly in the past decade despite many publications of strategies to reduce these complications. One reason for this may be that although CVS is recommended, it is often not attained. Secondly, only one or two of the three CVS criteria may be met as just two small windows are made and the liver surface is visualized but the gallbladder is not dissected off the cystic plate, and the surgeon terms it as the critical view of safety. If the CVS is achieved satisfactorily, it should ensure almost 100% safety from misidentification of the cystic duct from the CBD, CHD and RHD. However, it is still possible to injure a bile duct while attempting to dissect to the critical view, hence the recommendation to alter the approach if such difficulty is encountered.
An electronic questionnaire response from the Dutch surgical society found that 96.7% surgeons in the Netherlands employ the CVS and 80% of them document it by different methods. Sanjay et al. also found that 82% of the British and Irish upper gastrointestinal (GI) surgeons advocated the CVS technique (36). However, what is not known is how many surgeons actually practice this properly. The reasons which have been observed for it not being adopted are 3-fold:
- Achieving CVS is somewhat difficult and time consuming, as it involves more dissection before clipping or cutting the cystic artery and duct;
- An incomplete understanding of what the CVS is;
- Comfort with the infundibular technique which is comparatively easier and quick to achieve and works in the vast majority of patients.
One strategy would be to focus on training of young surgeons or residents to enhance awareness of the CVS and how to reliably achieve it. Yegiyants and Collins reported, in a retrospective review of 3,042 patients where residents were supervised by senior surgeons and had undergone training in the CVS method, only one BDI occurred (32). In the study by Nijssen of the 65 videos that were reviewed, GI surgeons failed to achieve the CVS in 22.4% of cases compared to 27.6% in residents supervised by an attending surgeon vs. 50% in non-GI surgeons (31). Sanjay et al. also reported in a study of 447 cases that 66% were done by trainees and the CVS was achieved in 87% of the cases (36). Moreover, 40% of the 447 cases were in patients with acute cholecystitis. Chen and associates evaluated the rate of achievement of the CVS in 57 residents over 101 LC’s before and after a lecture on safe cholecystectomy (54). Residents were empowered to carry out a time out once the CVS was obtained and to document with photographs and video. Mean CVS scores improved from 2.3 to 4.3 and the number with a CVS score >4 increased from 15.7% to 52%. These results suggest an appropriate intervention may increase the rate of achievement of the CVS.
Sages 6-step protocol for minimizing BDI during LC
In an effort to reduce the rate of biliary injury, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) launched the safe cholecystectomy task force with the goal of enhancing a universal culture of safety around this operation. A Delphi nominal group consensus process was undertaken to identify critical factors for safe surgical practice in laparoscopy which was completed by 160 SAGES committee members (55). Out of this effort arose a 6-step program of practices surgeons can adopt now to minimize these injuries which is shown in Table 3 and can be found online at www.sages.org/safe-cholecystectomy-program.
Table 3
| Steps | Strategies |
|---|---|
| 1 | Use the critical view of safety (CVS) method of identification of the cystic duct and cystic artery during laparoscopic cholecystectomy |
| 2 | Understand the potential for aberrant anatomy in all cases |
| 3 | Make liberal use of cholangiography or other methods to image the biliary tree intraoperatively |
| 4 | Consider an intraoperative time-out during laparoscopic cholecystectomy prior to clipping, cutting or transecting any ductal structures |
| 5 | Recognize when the dissection is approaching a zone of significant risk and halt the dissection before entering the zone. Finish the operation by a safe method other than cholecystectomy if conditions around the gallbladder is dangerous |
| 6 | Get help from another surgeon when the dissection or conditions are difficult |
A retrospective analysis to evaluate the feasibility and efficacy of this protocol was recently reported by Barot et al. for 173 laparoscopic cholecystectomies (56). After implementation of the 6-step program, they found that achievement of the CVS increased from 57.1% to 95.1% of cases and recognition of aberrant anatomy increased from 1.4% to 8.7%. The rate of intra-operative complications also decreased significantly from 5.7% to 0%. No differences were seen in the rate of use of IOC or conversion rate. These results suggest that adoption of these six measures has the potential to positively impact outcomes but requires further study on a broader scale.
SAGES is also in the process of developing a series of on-line didactic modules that further highlights safe principles of management. In addition, a multi-society consensus conference on prevention of BDI jointly sponsored by SAGES, the Americas Hepato-Pancreato-Biliary Association (AHPBA), International Hepato-Pancreato-Biliary Association (IHPBA), and Society for Surgery of the Alimentary Tract (SSAT) will be held on October 20, 2018 at the beginning of the American College of Surgeons meeting in Boston that will address the most critical questions on this topic.
Conclusions
The critical view of safety method of ductal identification is an effective approach according to the best evidence that exists to minimize the risk of BDI during LC. However, data from several studies suggest that it is still underutilized and misunderstood. Routinely incorporating this approach into practice as well as integrating the other strategies reviewed herein has the potential to improve outcomes for this common operation.
Acknowledgments
This work was supported by a grant from the Washington University Institute for Minimally Invasive Surgery and from Donald Sher.
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.12.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soper NJ, Stockmann PT, Dunnegan DL, et al. Laparoscopic cholecystectomy: the new gold standard. Arch Surg 1992;127:917-21. [Crossref] [PubMed]
- A prospective analysis of 1518 laparoscopic cholecystectomies. The Southern Surgeons Club. N Engl J Med 1991;324:1073-8. [Crossref] [PubMed]
- Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 1995;180:101-25. [PubMed]
- Giger UF, Michel JM, Opitz I, et al. Risk factors for perioperative complications in patients undergoing laparoscopic cholecystectomy: analysis of 22,953 consecutive cases from the Swiss Association of Laparoscopic and Thoracoscopic Surgery database. J Am Coll Surg 2006;203:723-8. [Crossref] [PubMed]
- Cuschieri A, Dubois F, Mouiel J, et al. The European experience with laparoscopic;cholecystectomy. Am J Surg 1991;161:385-7. [Crossref] [PubMed]
- Singh K, Ohri A. Difficult laparoscopic cholecystectomy: A large series from north India. Indian J Surg 2006;68:205-8.
- Singh K, Ohri A. Laparoscopic cholecystectomy - is there a need to convert? J Minim Access Surg 2005;1:59-62. [Crossref] [PubMed]
- Udwadia TE, Patil SU, Udwadia RT, et al. Laparoscopic cholecystectomy in India. Int Surg 1992;77:149-53. [PubMed]
- Rogers EA, Tang SJ, Porter J, et al. Suspected bile duct injuries and appropriate early referral can reduce chances of litigation. J Miss State Med Assoc 2011;52:275-7. [PubMed]
- Singh K, Singh R, Kaur M. Clinical reappraisal of vasculobiliary anatomy relevant to laparoscopic cholecystectomy. J Minim Access Surg 2017;13:273-9. [PubMed]
- Strasberg SM. Error traps and vasculo-biliary injury in laparoscopic and open cholecystectomy. J Hepatobiliary Pancreat Surg 2008;15:284-92. [Crossref] [PubMed]
- Benson EA, Page RE. A practical reappraisal of the anatomy of the extrahepatic bile ducts and arteries. Br J Surg 1976;63:853-60. [Crossref] [PubMed]
- Singh K, Ohri A. Anatomic landmarks: Their usefulness in safe laparoscopic cholecystectomy. Surg Endosc 2006;20:1754-8. [Crossref] [PubMed]
- Connor S, Garden OJ. Bile duct injury in the era of laparoscopic cholecystectomy. Br J Surg 2006;93:158-68. [Crossref] [PubMed]
- Machi J, Johnson JO, Deziel DJ, et al. The routine use of laparoscopic ultrasound decreases bile duct injury: a multicenter study. Surg Endosc 2009;23:384-8. [Crossref] [PubMed]
- Machi J, Oishi AJ, Tajiri T, et al. Routine laparoscopic ultrasound can significantly reduce the need for selective intraoperative cholangiography during cholecystectomy. Surg Endosc 2007;21:270-4. [Crossref] [PubMed]
- Buddingh KT, van Buuren L, Hulscher JB, et al. Intraoperative assessment of biliary anatomy for prevention of bile duct injury: A review of current and future patient safety interventions. Surg Endosc 2011;25:2449-61. [Crossref] [PubMed]
- Strasberg SM, Eagon CJ, Drebin J. The “Hidden Cystic Duct” syndrome and the infundibular technique of laparoscopic cholecystectomy—the danger of the false infundibulum. J Am Coll Surg 2000;191:661-7. [Crossref] [PubMed]
- Strasberg SM, Brunt LM. Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg 2010;211:132-8. [Crossref] [PubMed]
- Rawlings A, Hodgett SE, Matthews BD, et al. Single-incision laparoscopic cholecystectomy: initial experience with critical view of safety dissection and routine intraoperative cholangiography. J Am Coll Surg 2010;211:1-7. [Crossref] [PubMed]
-
The SAGES Safe Cholecystectomy Program - Singh R, Brunt LM. Dissection of the gallbladder off the liver to expose the lower one-third of the cystic plate. Asvide 2017;4:589. Available online: http://asvidett.amegroups.com/article/view/14159
- Sanford DE, Strasberg SM. A simple effective method for generation of a permanent record of the Critical View of Safety during laparoscopic cholecystectomy by intraoperative "doublet" photography. J Am Coll Surg 2014;218:170-8. [Crossref] [PubMed]
- Emous M, Westerterp M, Wind J, et al. Registering the critical view of safety: photo or video? Surg Endosc 2010;24:2527-30. [Crossref] [PubMed]
- Wauben LS, Goossens RH, van Eijk DJ, et al. Evaluation of protocol uniformity concerning laparoscopic cholecystectomy in the Netherlands. World J Surg 2008;32:613-20. [Crossref] [PubMed]
- Plaisier PW, Pauwels MM, Lange JF. Quality control in laparoscopic cholecystectomy: operation notes, video or photo print? HPB (Oxford) 2001;3:197-9. [Crossref] [PubMed]
- Stefanidis D, Chintalapudi N, Anderson-Montoya B, et al. How often do surgeons obtain the critical view of safety during laparoscopic cholecystectomy? Surg Endosc 2017;31:142-6. [Crossref] [PubMed]
- Nijssen MA, Schreinemakers JM, van der Schelling GP, et al. Improving critical view of safety in laparoscopic cholecystectomy by teaching interventions. J Surg Educ 2016;73:442-7. [Crossref] [PubMed]
- Deal SB, Stefanidis D, Brunt LM, et al. Development of a multimedia tutorial to educate how to assess the critical view of safety in laparoscopic cholecystectomy using expert review and crowd-sourcing. Am J Surg 2017;213:988-90. [Crossref] [PubMed]
- Deal SB, Stefanidis D, Telem D, et al. Evaluation of crowd-sourced assessment of the critical view of safety in laparoscopic cholecystectomy. Surg Endosc 2017;31:5094-100. [Crossref] [PubMed]
- Nijssen MA, Schreinemakers JM, Meyer Z, et al. Complications after laparoscopic cholecystectomy: A video evaluation study of whether the critical view of safety was reached. World J Surg 2015;39:1798-803. [Crossref] [PubMed]
- Yegiyants S, Collins JC. Operative strategy can reduce the incidence of major bile duct injury in laparoscopic cholecystectomy. Am Surg 2008;74:985-7. [PubMed]
- Avgerinos C, Kelgiorgi D, Touloumis Z, et al. One thousand laparoscopic cholecystectomies in a single surgical unit using the "critical view of safety" technique. J Gastrointest Surg 2009;13:498-503. [Crossref] [PubMed]
- Booij KA, de Reuver PR, Nijsse B, et al. Insufficient safety measures reported in operation notes of complicated laparoscopic cholecystectomies. Surgery 2014;155:384-9. [Crossref] [PubMed]
- Daly SC, Deziel DJ, Li X, et al. Current practices in biliary surgery: do we practice what we teach? Surg Endosc 2016;30:3345-50. [Crossref] [PubMed]
- Sanjay P, Fulke JL, Exon DJ. 'Critical view of safety' as an alternative to routine intraoperative cholangiography during laparoscopic cholecystectomy for acute biliary pathology. J Gastrointest Surg 2010;14:1280-4. [Crossref] [PubMed]
- Tsalis K, Antoniou N, Koukouritaki Z, et al. Open-access technique and "critical view of safety" as the safest way to perform laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech 2015;25:119-24. [Crossref] [PubMed]
- Heistermann HP, Tobusch A, Palmes D. Prevention of bile duct injuries after laparoscopic cholecystectomy. "The critical view of safety Zentralbl Chir 2006;131:460-5. [Crossref] [PubMed]
- Kaya B, Fersahoglu MM, Kilic F, et al. Importance of critical view of safety in laparoscopic cholecystectomy: a survey of 120 serial patients, with no incidence of complications. Ann Hepatobiliary Pancreat Surg 2017;21:17-20. [Crossref] [PubMed]
- Ludwig K, Bernhardt J, Steffen H, et al. Contribution of intraoperative cholangiography to incidence and outcome of common bile duct injuries during laparoscopic cholecystectomy. Surg Endosc 2002;16:1098-104. [Crossref] [PubMed]
- Flum DR, Dellinger EP, Cheadle A, et al. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA 2003;289:1639-44. [Crossref] [PubMed]
- Waage A, Nilsson M. Iatrogenic bile duct injury: a population-based study of 152 776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg 2006;141:1207-13. [Crossref] [PubMed]
- Törnqvist B, Strömberg C, Persson G, et al. Effect of intended intraoperative cholangiography and early detection of bile duct injury on survival after cholecystectomy: population based cohort study. BMJ 2012;345:e6457 [Crossref] [PubMed]
- Sheffield KM, Riall TS, Han Y, et al. Intraoperative cholangiography during cholecystectomy. JAMA 2013;310:812-20. [Crossref] [PubMed]
- Osayi SN, Wendling MR, Drosdeck JM, et al. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc 2015;29:368-75. [Crossref] [PubMed]
- Ishizawa T, Bandai Y, Ijichi M, et al. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg 2010;97:1369-77. [Crossref] [PubMed]
- Navez B, Ungureanu F, Michiels M, et al. Surgical management of acute cholecystitis: results of a 2-year prospective multicenter survey in Belgium. Surg Endosc 2012;26:2436-45. [Crossref] [PubMed]
- Schwaitzberg SD, Scott DJ, Jones DB, et al. Threefold increased bile duct injury rate is associated with less surgeon experience in an insurance claims database: more rigorous training in biliary surgery may be needed. Surg Endosc 2014;28:3068-73. [Crossref] [PubMed]
- Singh K, Matta H, Nain PS, et al. Modification of laparoscopic subtotal cholecystectomy. Surg Endosc 2011;25:2760. [Crossref] [PubMed]
- Strasberg SM, Pucci MJ, Brunt LM, et al. Subtotal Cholecystectomy-"Fenestrating" vs "Reconstituting" subtypes and the prevention of bile duct injury: definition of the optimal procedure in difficult operative conditions. J Am Coll Surg 2016;222:89-96. [Crossref] [PubMed]
- van Dijk AH, Donkervoort SC, Lameris W, et al. Short and long-term outcomes after a reconstituting and fenestrating subtotal cholecystectomy. J Am Coll Surg 2017;225:371-9. [Crossref] [PubMed]
- Way LW, Stewart L, Gantert W, et al. Causes and prevention of laparoscopic bile duct injuries. Analysis of 252 cases from a human factor and cognitive psychology perspective. Ann Surg 2003;237:460-9. [Crossref] [PubMed]
- Chuang KI, Corley D, Postlethwaite DA, et al. Does increased experience with laparoscopic cholecystectomy yield more complex bile duct injuries? Am J Surg 2012;203:480-7. [Crossref] [PubMed]
- Chen CB, Palazzo F, Doane SM, et al. Increasing resident utilization and recognition of the critical view of safety during laparoscopic cholecystectomy: a pilot study from an academic medical center. Surg Endosc 2017;31:1627-35. [Crossref] [PubMed]
- Pucher PH, Brunt LM, Fanelli RD, et al. SAGES expert Delphi consensus: critical factors for safe surgical practice in laparoscopic cholecystectomy. Surg Endosc 2015;29:3074-85. [Crossref] [PubMed]
- Barot TC, Canales A, Irving J, et al. SAGES 6-step Protocol for minimizing bile duct Injuries: A single center experience. SAGES 2016. Available online: https://www.sages.org/meetings/annual-meeting/abstracts-archive/sages-6-step-protocol-for-minimizing-bile-duct-injuries-a-single-center-experience/
Cite this article as: Singh R, Brunt LM. Critical view of safety—its feasibility and efficacy in preventing bile duct injuries. Ann Laparosc Endosc Surg 2018;3:2.