Robot-assisted surgery for rectal cancer: where do we stand?
Introduction
The last two decades have witnessed an increasingly acceptance of the laparoscopic approach to mid and lower rectal cancer. Laparoscopic total mesorectal excision (TME) is associated with better short-term outcomes than open surgery (1-3), while oncologic results are still under evaluation (4,5). However, laparoscopic surgery for extraperitoneal rectal cancer is technically challenging, being limited by the 2-dimensional view of the surgical field, and limited degrees of freedom of the surgical instruments.
The use of the robotic technology has been proposed to overcome these technical drawbacks, by providing a stable stereoscopic three-dimensional visualization of the surgical field, increased maneuverability of the surgical tools with consequent increased movement accuracy and precision, and improved ergonomics for the surgeon.
The impact of the robotic platforms on the outcomes of patients undergoing rectal resection and TME for rectal cancer is under debate despite a recently published randomized controlled multinational trial (6). The current evidence shows that this technology is safe and effective, with potential benefits in preserving bladder and sexual function. However, there is no randomized controlled trial supporting its superiority over laparoscopic surgery. In addition, the costs related to the use of the robot are higher than those of conventional laparoscopic TME (7).
The aim of this paper is to review the available evidence regarding outcomes after robot-assisted or conventional laparoscopic TME for mid and lower rectal cancer.
Literature search
The critical appraisal of the literature was performed searching the electronic PubMed/Medline databases and the Cochrane Library for articles published in English language between January 2010 and January 2018. The following medical subject headings (MeSH) and free-text words alone or in combination were used: “rectal cancer”, “robotic”, “robot-assisted”, “Da Vinci”, “laparoscopic”, “total mesorectal excision”. Reference lists from the included articles were manually checked, and additional studies were included when appropriate.
Study selection
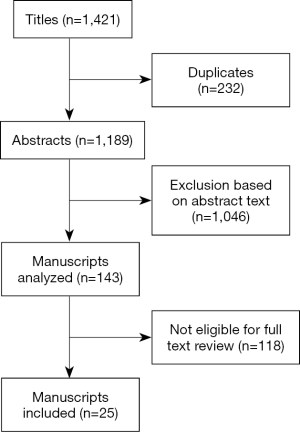
The literature search was performed independently by the two authors and was limited to articles published in English language. Reference lists from the included articles were manually checked and additional studies were included when appropriate. Studies were included if they reported on the use of the robot for the treatment of rectal cancer. When multiple publications on the same data from a single institution were retrieved, the most recent study was considered. The following data were extracted from each publication: year of publication, study design, number of patients included, postoperative morbidity and mortality, oncologic outcomes, costs. The study selection process is reported in Figure 1.
The evidence from the literature
To date, several studies comparing robotic and conventional laparoscopic TME have been published. Li et al. (8) have recently published a meta-analysis and systematic review of 17 case-control studies published between 2010 and 2016, including a total of 3,601 patients: 1,726 were treated with the use of the robot, while 1,875 underwent conventional laparoscopic rectal surgery. Only studies reporting on at least one of the following outcomes were considered: operative time, estimated blood loss, conversion to open surgery, length of postoperative hospital stay, resumption of gastrointestinal function, postoperative complications, reoperation rate, number of harvested lymph nodes, length of the distal resection margin, rate of involved circumferential resection margins, local recurrence and 3-year overall survival.
All 17 studies are retrospective. Among them, 15 are single-institution series. The samples size varies largely between 40 and 1,019 patients.
Operative time, estimated blood losses and conversion to open surgery
Most studies reported significantly longer operative time of robot-assisted surgery, while 3 studies did not find any significant difference and 1 study showed shorter operative time in the robotic group. Pooling all data, robot-assisted TME took 57 min more than laparoscopic TME.
No significant differences were reported in most studies comparing the two approaches: only 3 studies demonstrated lower blood losses during robotic surgery. As a consequence, the pooled analysis failed to show any difference between robot-assisted and conventional laparoscopic TME.
Twelve studies reported on the conversion rate to open surgery. The pooled data analysis revealed a lower incidence of conversion for robot-assisted TME.
Length of hospital stay, first passing flatus, 30-day reoperation rate and overall complication rate
Among the 16 studies that evaluated the length of hospital stay, 13 studies reported no significant differences, 2 showed shorter hospital stay after robotic TME and 1 shorter hospital stay after laparoscopic TME. The pooled data analysis showed similar length of hospital stay regardless of the surgical approach.
Bowel function recovered in a similar way in the two groups of patients, with a trend that did not reach the statistical significance towards a quicker time to first passing flatus after robotic surgery.
Similar rates of 30-day reoperations and overall morbidity were reported in the studies included in the meta-analysis. The subgroup analysis of the complications showed a higher rate of bowel obstruction and a lower rate of urinary retention after robotic TME, while similar results were reported in terms of anastomotic leaks, bleeding and wound infection.
Number of harvested lymph nodes, distal and circumferential margin status, local recurrence and overall survival at 3 years
All but one study reported a similar number of lymph nodes harvested in the specimen. Consequently, no significant differences were found by pooling the study data. Similarly, there were no differences in length of distal margin and in the rate of involved circumferential resection margins.
Data regarding long-term oncologic outcomes are limited, with only a few studies investigating local recurrence and overall survival at 3 years after surgery. All studies showed similar oncologic outcomes after robot-assisted or conventional laparoscopic TME.
Costs
Several comparative studies have focused on costs after laparoscopic or robotic rectal resection (9-13). All studies have reported higher costs in the robotic group. For instance, Keller et al. (12) reported the results of the evaluation of a national inpatient database. The authors included 1,838 laparoscopic resections and 105 robot-assisted resections. Robotic surgery was associated with significantly higher total costs (+$5,107).
In 2017, the results of two RCTs comparing robot-assisted and conventional laparoscopic rectal resection for cancer became available (Table 1) (6,14).
Table 1
| Reference | Country | Number of patients | Operative time | Conversion to open surgery | Morbidity | Length of hospital stay |
|---|---|---|---|---|---|---|
| Jayne et al. (6) | International | 234 lap, 237 robot | Robot > lap (37.5 min) | Robot = lap | Robot = lap | Robot = lap |
| Kim et al. (14) | South Korea | 73 lap, 66 robot | Robot > lap (112 min) | Robot = lap | Robot = lap | Robot = lap |
Lap, laparoscopic; Robot, robot-assisted.
The RObotic vs. LAparoscopic Resection for Rectal cancer (ROLARR) RCT (6) is an international (United Kingdom, Italy, Denmark, United States, Finland, South Korea, Germany, France, Australia and Singapore) multicenter randomized, unblinded, parallel-group trial that compares robot-assisted and conventional laparoscopic curative resection (high anterior resection, low anterior resection or abdominoperineal resection) for rectal cancer with a distal extent within 15 cm from the anal verge. Each surgeon was asked to perform at least 30 minimally invasive (of which at least 10 robotic and 10 laparoscopic) rectal resections for cancer before being part of the trial. Exclusion criteria were benign rectal lesions, anal cancer, distant metastases, locally advanced rectal cancer requiring en bloc multivisceral resection, and synchronous colorectal cancers. Randomization was performed on a 1 to 1 basis. The primary endpoint of this RCT was the conversion rate to open surgery. Secondary end points were pathological positive circumferential margin rate, quality of mesorectal excision, intraoperative complications, 30-day and 6-months postoperative morbidity, 30-day mortality, bladder and sexual function assessed by using the International Prostate Symptom Score (IPSS), the International Index of Erectile Function (IIEF), and the Female Sexual Function Index (FSFI) before the operation and 6 months after surgery. A cost analysis was also undertaken. The sample size was 400 patients in order to detect a decrease in the rate of conversion to open surgery from 25% in the conventional laparoscopic arm (based on the MRC CLASICC data) to 12.5% in the robotic arm, allowing for 16% attrition. All analyses were performed on an intention-to-treat basis.
Between January 2011 and September 2014, a total of 471 patients were randomized: 234 to conventional laparoscopic rectal resection and 237 to robotic rectal surgery. Low anterior resection was performed in 317 patients, with an abdominoperineal resection being needed in 97 patients. Baseline patients’ characteristics, types of surgery and use of neoadjuvant (chemo) radiation therapy were similar in both groups.
Operative time was about 38 minutes longer in the robotic arm. Regarding the primary outcome, conversion to open surgery occurred in 12.2% of conventional laparoscopic surgeries and in 8.1% of robot-assisted surgeries: this difference was not statistically significant. The multilogistic regression showed that obese patients, male patients and patients undergoing low anterior resection were at a significantly higher risk of conversion. Intraoperative complication rates after laparoscopic or robotic rectal surgery were similar: 14.8% vs. 15.3%, P=0.94.
Pathological outcomes were similar: mean number of lymph nodes harvested was 24.1 and 23.2 after laparoscopic and robotic rectal resection; circumferential resection margins were positive in 6.2% of laparoscopic patients and in 5.1% of robotic patients (P=0.56). Also quality of the mesorectal dissection was not affected by the type of surgical approach.
Postoperative course was similar in the two groups. In particular, no significant differences were observed in the length of hospital stay, 30-day morbidity (31.7% vs. 33.1%, P=0.84), 60-day morbidity (16.5% vs. 14.4%, P=0.25) and 30-day mortality (0.9% vs. 0.8%). Anastomotic leak occurred in 9.9% of patients undergoing laparoscopic surgery and in 12.2% of patients treated with the robotic platform.
The functional evaluation at 6 months after the operation failed to show any statistically significant difference in terms of IPSS, IIEF and FSFI scores.
Lastly, the cost analysis demonstrated that robotic surgery was significantly more expensive than conventional laparoscopic surgery (+£980 or $1,132), mainly due to the prolonged operative time and the use of dedicated surgical tools.
Similar results have been published by Kim et al. (14) They conducted a phase II RCT enrolling cT1–3NxM rectal cancer patients between February 2012 and March 2015. Primary outcome was the TME specimen quality. Secondary outcomes were distal and circumferential resection margin status, number of lymph nodes harvested in the specimen, postoperative morbidity, resumption of bowel function and quality of life. The sample size was calculated based on the rate of incomplete TME of 9% after laparoscopic resection. A similar rate was expected in the robotic-assisted group: considering a drop rate of 10%, 73 patients in each group were needed to estimate the rate of incomplete TME.
A total of 163 rectal cancer patients were included: 81 were randomized to robot-assisted TME and pelvic autonomic nerve preservation and 82 to conventional laparoscopic resection. Patients were similar in both groups, with no significant differences in terms of demographic characteristics, use of preoperative therapy and type of surgical procedure.
Robot-assisted surgery took significantly longer than laparoscopic surgery (340 vs. 228 min, P<0.0001) and the estimated blood loss were higher (100 vs. 50 mL, P<0.0001). No blood transfusions were required. Intraoperative complications occurred in 7.6% of robotic procedures and in 4.1% of laparoscopic procedures (P=0.647). Conversion rate to open surgery did not differ between robotic and laparoscopic group (1.5% vs. 0%).
Postoperative pathology did not show any significant difference in the quality of TME specimen, and in the rate of positive circumferential resection margins. The only significant difference was related to the higher mean number of lymph nodes harvested after robotic TME (18 vs. 15, P=0.04).
Short-term postoperative outcomes were similar in the two groups, with no significant differences in postoperative pain, resumption of gastrointestinal function and diet and length of postoperative hospital stay. A higher rate of overall postoperative complications (34.8% vs. 23.3%) and anastomotic leak (12.1% vs. 6.8%) was observed in the robotic group; however, these differences did not reach the statistical significance.
The authors did not find significant differences in quality of life measured with QLQ-CR38 questionnaire; however, sexual function at one year after surgery was better after robotic surgery.
Discussion
The use of robotic technology in rectal cancer surgery has gained worldwide popularity during the last ten years. The supporters of the robot-assisted surgery claim lower risk of conversion to open surgery, more accurate dissection of the surgical planes, higher rate of mesorectal fascia preservation and lower injury to the autonomic pelvic nerves than standard laparoscopic surgery (15). However, these potential benefits mostly derive from the results of non-randomized controlled trials, while two recent large RCTs have shown no clear advantages provided by the use of robotic technology, reporting comparable outcomes between robotic and standard laparoscopic rectal resection.
In addition to the recently published results of the ROLARR and Korean RCTs, two recent investigations have questioned the validity of the potential benefits of the robotic surgery reported in non-randomized studies (16,17). Criss et al. (16) used the CMS Open Payments website to search for “Intuitive Surgical Incorporated (ISI)”, aiming at analyzing potential correlations between financial relationships with the industry and scientific quality of the literature. The authors identified the top 20 earners in 2015 and their scientific publications on PubMed. Exclusion criteria from this investigation were studies not conducted in the US, case reports, and studies with less than 5 patients included. A total of 37 studies were considered: 27 had “positive” conclusions in favor of the DaVinci robot, while only 1 (3%) reported negative results. A total of 6 (16%) studies were sponsored by ISI and all were “positive”. This pilot study suggests a word of caution in the interpretation of the published outcomes in patients treated with the aid of the robotic technology, showing a potential influence of industry sponsoring on the study conduction and publication.
Patel et al. have recently (17) investigated the accuracy of the disclosure of Intuitive Inc. payments in self-declared conflict of interest (COI) statements in robotic studies and its correlation with the published results. The authors included studies published in 2015 that were designed to assess the outcomes of the DaVinci platform in a clinical setting, with at least one American author. The web search was performed in EMBASE and MEDLINE databases. Even in this study, authors’ payments were checked by using the “Open Payments” database. A total of 458 papers were included in the analysis and 303 studies had at least one author who had received payments from the industry. Interestingly, only 63 (21%) studies with authors receiving payments declared this financial relationship in a COI statement, even though 29 (46%) of them only partially declared it.
Among the 287 studies that did not declare funding in the COI, in 183 (64%) cases at least one author was found to have received payments from the industry. Similarly, at least one author of 57 out of 108 papers (53%) that did not report the COI statement received funding from Intuitive. The order of the authors in the manuscript was associated with funding: first author 17.8%, middle 19.9%, last author 47% (P<0.001). Overall, the use of robotic technology was recommended by 79.5% of studies. On multivariate analysis, lack of declaration of payments and discrepancy between declaration and actual COI were independently associated with “positive” results in favor of robotic surgery.
Conclusions
The current evidence does not show clear benefits associated with the use of robotic surgery for the treatment of rectal cancer. Furthermore, the current literature seems to be heavily influenced by funding from industry sources. In the absence of level 1 unbiased evidence proving the alleged benefits of robotic assisted surgery, widespread acceptance of such an approach in rectal cancer should not be supported.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.03.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637-45. [Crossref] [PubMed]
- van der Pas MH, Haglind E, Cuesta MA, et al. COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Arezzo A, Passera R, Scozzari G, et al. Laparoscopy for rectal cancer reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Surg Endosc 2013;27:1485-502. [Crossref] [PubMed]
- Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes. The ACOSOG Z6051 randomized clinical trial. JAMA 2015;314:1346-55. [Crossref] [PubMed]
- Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer. The ALaCaRT randomized clinical trial. JAMA 2015;314:1356-63. [Crossref] [PubMed]
- Jayne D, Pigazzi A, Marshall H, et al. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA 2017;318:1569-80. [Crossref] [PubMed]
- Kim CW, Baik SH, Roh YH, et al. Cost-effectiveness of robotic surgery for rectal cancer focusing on short-term outcomes: a propensity score-matching analysis. Medicine (Baltimore) 2015;94:e823 [Crossref] [PubMed]
- Li X, Wang T, Yao L, et al. The safety and effectiveness of robot-assisted versus laparoscopic TME in patients with rectal cancer: A meta-analysis and systematic review. Medicine (Baltimore) 2017;96:e7585 [Crossref] [PubMed]
- Morelli L, Guadagni S, Lorenzoni V, et al. Robot-assisted versus laparoscopic rectal resection for cancer in a single surgeon’s experience: a cost analysis covering the initial 50 robotic cases with the da Vinci Si. Int J Colorectal Dis 2016;31:1639-48. [Crossref] [PubMed]
- Bertani E, Chiappa A, Biffi R, et al. Assessing appropriateness and quality-of-life short-term outcomes employing different treatment approaches. Int J Colorectal Dis 2011;26:1317-27. [Crossref] [PubMed]
- Baek SJ, Kim SH, Cho JS, et al. Robotic versus conventional laparoscopic surgery for rectal cancer: a cost analysis from a single institute in Korea. World J Surg 2012;36:2722-9. [Crossref] [PubMed]
- Keller DS, Senagore AJ, Lawrence JK, et al. Comparative effectiveness of laparoscopic versus robot-assisted colorectal resection. Surg Endosc 2014;28:212-21. [Crossref] [PubMed]
- Ielpo B, Duran H, Diaz E, et al. Robotic versus laparoscopic surgery for rectal cancer: a comparative study of clinical outcomes and costs. Int J Colorectal Dis 2017;32:1423-9. [Crossref] [PubMed]
- Kim MJ, Park SC, Park JW, et al. Robot-assisted Versus Laparoscopic Surgery for Rectal Cancer: A Phase II Open Label Prospective Randomized Controlled Trial. Ann Surg 2018;267:243-51. [Crossref] [PubMed]
- Broholm M, Pommergaard HC, Gögenür I. Possible benefits of robot-assisted rectal cancer surgery regarding urological and sexual dysfunction: a systematic review and meta-analysis. Colorectal Dis 2015;17:375-81. [Crossref] [PubMed]
- Criss CN, Gadepalli SK. Sponsoring surgeons; an investigation on the influence of the da Vinci robot. Am J Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Patel SV, Yu D, Elsolh B, et al. Assessment of Conflicts of Interest in Robotic Surgical Studies: Validating Author's Declarations With the Open Payments Database. Ann Surg 2017. [Epub ahead of print].
Cite this article as: Allaix ME, Fichera A. Robot-assisted surgery for rectal cancer: where do we stand? Ann Laparosc Endosc Surg 2018;3:21.