Robotic approach for rectal cancer, is the best approach?
Introduction
Minimally invasive surgery (MIS) is considered one of the leading advances in rectal cancer surgery (RCS) and has been exponentially utilized as a surrogate for open RCS (ORCS). Nevertheless, two recent randomized studies, ALaCaRT and ACOSOG Z6051 (1,2), have failed to show non-inferiority of the laparoscopic RCS (LRCS) against ORCS in terms of conversion and circumferential radial margin (CRM) positivity rates and raised concerns regarding the implementation of the laparoscopy in RCS settings.
Total mesorectal excision (TME), a technique first described by Heald et al. in 1982 (3), is currently the mainstay of oncologic resection in RCS. Achieving a complete resection of the mesorectum requires meticulous dissection of the embryologic planes within the pelvis, where the rectum is in close proximity to vasculature and nerves. The incorporation of robotic systems into RCS have been anticipated to overcome the limited dexterity associated with laparoscopic surgery by introducing endo-wristed equipment that offers better articulation, tremor-free retraction and a stable, surgeon-driven camera platform. However, robotic surgery generally takes longer to perform than a laparoscopic approach and is more expensive. Both of these factors raise concerns about whether this novel technique offers surgeons and patients any advantages over laparoscopy in RCS. In this review, we will discuss the use of robotic systems in light of the most recent advances in RCS as well as the results of an eagerly anticipated study called the RObotic versus LAparoscopic Resection for Rectal Cancer (4) trial, in which the authors compared da Vinci robotic system(Intuitive Surgical Systems, Sunnyvale, CA, USA) to conventional laparoscopic surgery.
Methods
We performed a comprehensive search on PubMed using the key words “total mesorectal excision,” “robotic proctectomy,” “robotic surgery,” “laparoscopic surgery,” and “robotic rectal cancer.”. All meta-analyses, randomized trials, prospective and retrospective cohorts, and reviews published between 2000 and 2017 in the English language were considered. We have also included unpublished data from our center where specified.
Patient selection and preoperative workup
Patient selection for robotic RCS (RRCS) is one of the most important steps to optimizing surgical outcomes. A number of factors may restrict access to the pelvic cavity, including previous abdominal operations, male gender, low-lying tumors and obesity (BMI >30), although these are not absolute contraindications. Moreover, obese patients may benefit from undergoing RRCS versus LRCS as the former was associated with a quicker return of bowel function (median 3 vs. 4 days) and a shorter hospital stay (median 6 vs. 7 days) in one study (5).
Patient history and physical examination are essential parts of the preoperative workup as well as a full colonoscopy to rule out the presence of any potential synchronous lesions. Preoperative oral antibiotics and mechanical bowel preparation are recommended to decrease the risk of surgical site infection. Thirty to 60 minutes prior to incision, intravenous antibiotics should be given to the patient as indicated in the Surgical Care Improvement Project (6) to help maintain optimal levels during surgery. Preoperative heparin prophylaxis and compressive devices decrease the risk of deep venous thrombosis.
Robotic procedure
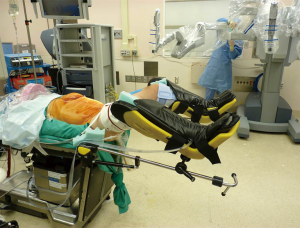
Prior to incision, the rectum is irrigated with saline, and a Foley catheter and orogastric tube are inserted. The patient is placed in a modified lithotomy position to ease access to the anus during intraoperative colonoscopy and to facilitate circular stapler placement. It also allows the surgical team to easily manipulate the upper abdomen between the patient’s legs. Yellow sponge fins or padded stirrups are used to prevent peroneal nerve injury. A gel pad is placed on the operating table to provide decubitus support. Tape is placed around the patient’s chest to help stabilize him or her and reduce pressure on the lower limbs when the operating table is tilted. Finally, both arms are tucked at the patient’s sides (Figure 1).
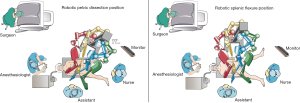
Intraoperative sigmoidoscopy is routinely performed to locate the tumor and to evaluate the integrity of the anastomosis. Two monitors are placed on each side of the operating table to assist the surgical team.
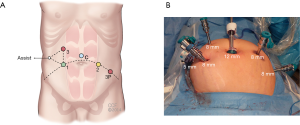
Port placement is crucial because the surgeon must have enough space in which to manipulate the robotic arms, which can otherwise collide with one another. Port placement (Figure 2) is generally guided by the surgeon’s experience. In our practice, five to six ports are commonly used, including one camera port and one assistant port.
When using S or Si generation, a 12-mm camera port is placed in the supraumbilical area with an open technique. An 8-mm port is placed in the left lower quadrant lateral to the umbilicus 10 cm apart; two additional 8-mm ports are placed—one in the right upper and one in the right lower quadrant. One assistant port is inserted on the right lateral to the robotic ports in equal distance from the right upper and lower quadrant trocars. A right upper quadrant robotic port (port 3) is used for splenic flexure mobilization and left colectomy. An additional robotic port (port 3P) is placed in the left-mid abdomen, lateral to the edge of the rectus muscle. This port, 3P, is used for pelvic dissection (anterior resection, low anterior resection, abdominoperineal resection procedures). The assistant port is used for bowel retraction, suction and irrigation purposes (Figure 3).
The operation begins by establishing pneumoperitoneum followed by the laparoscopic exploration of the tumor site and the rest of the abdomen. Once additional ports are entered, the patient is positioned in a right tilt along with a slight Trendelenburg so that the small bowel loops and the cecum can be removed from the pelvis. A medial-to-lateral dissection along with embryologic planes between the mesocolon and the retroperitoneum is made with monopolar cautery and scissors. The inferior mesenteric artery is identified, skeletonized and subsequently ligated with Hem-o-Lock clips (Weck Surgical Instruments, Teleflex Medical, Durham, NC, USA) 1 to 1.5 cm away from the aorta. The inferior mesenteric vein is also identified at the inferior border of the pancreas, isolated and clipped. Further dissection is carried out between the mesocolon and Gerota’s fascia along the Toldt line.
After the gastrocolic and splenocolic ligaments are divided, the lesser sac is entered and the left colon is mobilized. Once a medial-to-lateral dissection is performed and the splenic flexure is mobilized, the right upper quadrant robotic arm is switched from the right side to the left side. Pelvic dissection is then started. A mesorectal excision is made within an avascular plane between the mesorectal envelope and endopelvic parietal fascia, starting at the level of the sacral promontory. Dissection is continued toward the levator muscles, protecting the hypogastric plexuses while preserving the entirety of the mesorectum. Preferentially, the rectum is divided with a reticulating robotic stapling device.
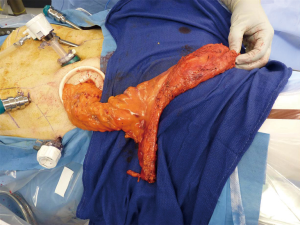
At this particular point, a rare technique may be utilized in which the rectum can be transected under direct vision with a cautery hook. A purse string suture is then placed around the rectal cuff and subsequently anastomosis is established with a circular stapler. The specimen is exteriorized from the right lower quadrant incision with a wound protector, where an ileostomy will potentially be placed (Figure 4). Natural orifice specimen extraction through the anus has also been described in patients undergoing RCS.
A hybrid technique, also called a laparoscopic-assisted robotic technique, eliminates the need to reposition the robotic arms, thereby reducing the overall operative time. The robotic system is designed to work better in confined places such as the pelvis. Thus, the transition to robotic pelvic dissection for TME after laparoscopic mobilization of the splenic flexure facilitates dissection and prevents any disruption at the flow of the operation.
TME: transition to robotic era
In the past ten years, some studies assessing LRCS have been published, allowing surgeons to gain a better and more concrete understanding of clinical and oncological outcomes. Initially, the MRC CLASICC trial (7) reported a 34% conversion rate and increased radial margin involvement compared to ORCS. However, during the study’s 3-year follow-up period, there was no increase in local recurrences or any changes in disease-free survival and overall survival (8).
LRCS gained more popularity with the COREAN (9) and COLOR II (10) randomized controlled trials, which reported surgically acceptable outcomes and no differences in oncologic quality of the specimen between ORCS and LRCS. In fact, LRCS was associated with better short-term postoperative findings. Moreover, three year follow-up in the COLOR II trial indicated that locoregional recurrence (5% vs. 5%), disease-free survival (74.8% vs. 70.8%) and overall survival rates (86.7% vs. 83.6%) were comparable between laparoscopic and ORCS (11), respectively.
On the other hand, the ALaCaRT (1) and ACOSOG Z6051 (2) trials further changed the approach to LRCS and failed to show noninferiority of laparoscopy against ORCS. The results of these two trials increased the skepticism about whether laparoscopy is preferential to ORCS in terms of mesorectal completeness and clear CRMs.
While discussions about laparoscopic TME continue, RRCS has emerged as a novel technique in RCS. Numerous trials, case-control studies and meta-analyses have compared RRCS with LRCS. Early reports depicted favorable or comparable results for RRCS (12-17). Although robotic surgery was associated with a longer operative time and higher costs, tumor-free CRM rates were lower and there was fewer postoperative complications and conversions. In their propensity score match analysis, Kang et al. (18) reported that patients who underwent RRCS were able to resume their bowel habits earlier, had a shorter length of stay and lower visual analog scale pain scores in the postoperative period.
A number of meta-analyses have been published in recent years, allowing us to evaluate this novel approach in RCS in more depth. Among these, Memon et al. (19) compared eight studies and concluded that RRCS was associated with fewer conversions to open, which is congruent with other studies. Similarly, Cui et al. (20) reviewed nine papers and found that RRCS was superior to LRCS in terms of conversions, intraoperative blood loss, hospital stay and short-term complications although operative time was longer.
The surgical community has eagerly awaited the results of RObotic versus LAparoscopic Resection for Rectal Cancer (4) trial, which concluded that RRCS did not differ from LRCS with respect to conversions, CRM positivity, number of harvested lymph nodes, quality of life and postoperative complications. Even though implementation of a non-standardized operating technique and surgeons with different levels of experience in robotic surgery may affect the outcomes, in the subgroup analysis, robotic surgery was found to be beneficial in male patients.
These recent meta-analyses seem to suggest that RRCS provides at least similar to relatively better short-term surgical outcomes and may offer new aspects in RCS. Details of the clinical and oncologic outcomes of the selected reports are summarized in Tables 1 and 2, respectively.
Table 1
| Author | Year | Number of patients | Type of surgery | Operative time, min | EBL, mL | LOS, days | Overall complication rate (%) | Conversion rate (%) |
|---|---|---|---|---|---|---|---|---|
| Kim et al. (21) | 2010 | 100 | RRCS | 385±102 | NA | 11.7±6.7 | 20 | 2 |
| 100 | LRCS | 297±83 | NA | 14.4±10 | 27 | 3 | ||
| Baek et al. (22) | 2011 | 41 | RRCS | 296 [150–520] | 200 | 6.5 | 22 | 7.3 |
| 41 | LRCS | 315 [174–584] | 300 | 6.6 | 26.8 | 22 | ||
| D’Annibale et al. (23) | 2013 | 50 | RRCS | 270 [240–315] | NA | 8 [7–11] | 10 | 0 |
| 50 | LRCS | 280 [240–350] | NA | 10 [8–14] | 22 | 12 | ||
| Kang et al. (18) | 2013 | 165 | RRCS | 309±115 | 133 | 10.8±5.5 | 20.6 | 0.6 |
| 165 | LRCS | 277±81 | 140 | 13.5±9.2 | 27.6 | 1.8 | ||
| 165 | ORCS | 252±88 | 275 | 16±8.6 | 24.8 | NA | ||
| Yoo et al. (24) | 2015 | 44 | RRCS | 316±65 | 239 | 11.4±5 | 38.6 | 0 |
| 26 | LRCS | 286±51 | 215 | 11±6 | 26.9 | 0 | ||
| ROLARR (4) | 2017 | 237 | RRCS | 295±88 | NA | 8±5.8 | 33.1 | 8.1 |
| 234 | LRCS | 261±83 | NA | 8.2±6 | 31.7 | 12.2 |
EBL, estimated blood loss; LOS, length of stay; RRCS, robotic rectal cancer surgery; LRCS, laparoscopic rectal cancer surgery; ORCS, open rectal cancer surgery; NA, not available.
Table 2
| Author | Type of surgery | CRM positivity (%) | DRM, cm | Harvested lymph node number | DFS (%) | OS (%) |
|---|---|---|---|---|---|---|
| Kim et al. (21) | RRCS | 3 | 2.7±1.9 | 14.7±9.7 | NA | NA |
| LRCS | 2 | 2.6±1.8 | 16.6±9.1 | NA | NA | |
| Baek et al. (22) | RRCS | 4.9 | 3.6 | 13.1 | NA | NA |
| LRCS | 2.4 | 3.8 | 16.2 | NA | NA | |
| D’Annibale et al. (23) | RRCS | 0 | 3 | 16.5 | NA | NA |
| LRCS | 12 | 3 | 13.8 | NA | NA | |
| Kang et al. (18) | RRCS | 4.2 | 1.9±1.4 | 15±9.4 | 83.5 | NA |
| LRCS | 6.7 | 2±1.4 | 15.6±9.1 | 81.9 | NA | |
| ORCS | 10.3 | 2.2±1.7 | 17.4±10.9 | 79.7 | NA | |
| Yoo et al. (24) | RRCS | 9.1 | 1.3±0.9 | 13.9±9 | 76.7 | 95.2 |
| LRCS | 19.2 | 1.67±3 | 21.4±15.7 | 75 | 88.5 | |
| ROLARR (4) | RRCS | 5.1 | NA | 23.2 | NA | NA |
| LRCS | 6.3 | NA | 24.1 | NA | NA |
CRM, circumferential resection margin; DRM, distal resection margin; DFS, disease free survival; OS, overall survival; NA, not available; RRCS, robotic rectal cancer surgery; LRCS, laparoscopic rectal cancer surgery; ORCS, open rectal cancer surgery.
Correlation of experience and cost
Increased operating time and cost are the main drawbacks for RRCS. A recent study from our institution indicated that RRCS costs on average 30% more than ORCS and LRCS, which included the surgeon’s learning curve and multiple procedures but not any additional costs associated with readmissions and reoperations during the study period (25). Similarly, in the ROLARR trial, robotic surgery cost $1,131 more per operation than laparoscopy (4). Robotic surgery is more expensive due to fixed costs such as those for the operating theater and disposable robotic arms. Interestingly, Morelli et al. (26) reported that when the fixed costs were excluded, the overall costs of RRCS and LRCS were similar, especially as surgeons experience increased. Unpublished data from our institution suggests that is possible to minimize the cost difference between open and robotic restorative proctectomies over the long term by using a cost-conscious approach (using cautery and clips instead of vessel sealing device etc.) and increasing surgeon experience, both of which cut costs associated with operative theater, ileostomy closures and surgery-related readmissions and reoperations (Table 3). Of course, short-term data on robotic surgery show increased costs, as one would expect. Nevertheless, if more long-term comparisons show comparable economic outcomes, the value of RRCS may become clearer.
Table 3
| Years | Total (N=100) | Open proctectomy (N=68) | Robotic proctectomy (N=32) | P value | |||
|---|---|---|---|---|---|---|---|
| n | Cost | n | Cost | ||||
| 2011–2012 | 27 | 22 | 1±0.4 | 5 | 1.5±0.5 | 0.02 | |
| 2013 | 38 | 26 | 1±0.8 | 12 | 0.96±0.3 | 0.14 | |
| 2014 | 35 | 20 | 1±0.6 | 15 | 1.2±0.6 | 0.10 | |
| 2017* | 100 | 68 | 1±0.5 | 32 | 1±0.4 | 0.96 | |
Asterisk indicates cumulative costs after 33 months including loop ileostomy closures, readmission and reoperations. Actual cost values are not presented due to our institution’s cost privacy policy. Presented cost value was the ratio between the actual mean direct costs with standard deviation divided by actual mean direct cost of the open surgery.
Learning curve
In order to master a new technical skill, it is vital to practice it through continuous repetition. Jiménez-Rodríguez et al. (27) and Sng et al. (28) proposed similar 3-layered learning curves for robotic-assisted surgery for rectal cancer, which are: attaining skills, strengthening technique and gaining expertise. As surgeons’ robotic skills improve, so does their ability to manipulate the console and utilize instruments, which subsequently leads to better surgical outcomes. Mastering robotic surgery skills may lead to less consumption of surgical equipment and lower surgery costs.
Operating time is frequently used as a primary predictor in learning curve studies to assess skill acquisition. On average, 9 to 35 operations are necessary in order for surgeons to achieve the first phase of the learning curve (27-29) in RRCS whereas the learning curve of the LRCS varies 40 to 50 cases regarding operative time (30-32). Park et al. further compared the learning curves of the RRCS and LRCS, which showed no difference (33). Nevertheless, gaining competence in handling a host of possible intraoperative complications is also important to consider when interpreting learning curves. Thus, relying on operative time as a measure of learning is still controversial.
Robotic rectal surgery in challenging cases
MIS is a more technically challenging procedure in obese patients where the maneuverability of the rigid laparoscopic instruments is highly restricted due to excessive fat tissue in the intra-abdominal area. Robotic surgery has the proposed benefit of increasing articulation in these patients. Our institutional data comparing RRCS and LRCS in obese patients who are case-matched regarding age, gender, body mass index (34.9 vs. 35.2 kg/m2, respectively), comorbidities, surgical and tumor characteristics showed that those who underwent RRCS experienced earlier return of bowel function (3 vs. 4 days, median, respectively) and a shorter hospital stay (6 vs. 7 days, respectively) (5) (Table 4).
Table 4
| Variable | RP group (n=29) | LP group (n=27) | P value |
|---|---|---|---|
| Intraoperative outcomes | |||
| Operative blood loss (mL), mean (SD) | 434.0 (612.4) | 339.4 (271.9) | 0.68 |
| Intraoperative transfusion, n (%) | 6 (20.7) | 5 (18.5) | 0.84 |
| Intraoperative complication, n (%) | 2 (6.9) | 0 (0.0) | 0.49 |
| Ureter injury | 0 | 0 | |
| Conversion to open surgery, n (%) | 1 (3.4) | 5 (18.5) | 0.09 |
| Operative time (min), mean (SD) | 329.0 (102.2) | 294.6 (81.1) | 0.13 |
| Oncologic outcomes | |||
| Proximal margin (cm), mean (SD) | 17.4 (6.2) | 21.6 (9.3) | 0.09 |
| Distal margin (cm), mean (SD) | 3.9 (2.1) | 3.2 (2.0) | 0.17 |
| LN total, mean (SD) | 25.5 (14.0) | 21.8 (9.6) | 0.52 |
| Radial margin involved (≤1 mm), n (%) | 2 (6.9) | 2 (7.4) | 0.99 |
| Postoperative outcomes | |||
| Bowel movement (days), mean (SD) | 3.2 (1.9) | 4.3 (2.3) | 0.01 |
| Oral regimen (days), mean (SD) | 3.7 (2.4) | 4.6 (3.4) | 0.59 |
| Hospital stay (days), mean (SD) | 6.4 (4.2) | 8.4 (4.4) | 0.02 |
| Anastomotic leak, n (%) | 0 | 0 | |
| Overall SSI, n (%) | 4 (13.8) | 4 (14.8) | 0.99 |
| Wound disruption, n (%) | 2 (6.9) | 2 (7.4) | 0.99 |
| Sepsis, n (%) | 1 (3.4) | 1 (3.7) | 0.99 |
| Ileus, n (%) | 4 (13.8) | 8 (29.6) | 0.15 |
| Urinary tract infection, n (%) | 1 (3.4) | 1 (3.7) | 0.99 |
| Urinary retention, n (%) | 7 (24.1) | 5 (18.5) | 0.61 |
| Atelectasis, n (%) | 2 (6.9) | 0 (0.0) | 0.49 |
| Re-operation, n (%) | 2 (6.9) | 2 (7.4) | 0.99 |
| Re-admission, n (%) | 4 (13.8) | 6 (22.2) | 0.41 |
| Mortality, n (%) | 0 | 0 |
RP, robotic proctectomy; LP, laparoscopic proctectomy; LN, lymph node; SSI, surgical site infection.
Abdominoperineal resection (APR) is still the preferred method for low-lying cancers that invade the sphincter muscles, but it requires high maneuverability within the pelvis. Moghadamyeghaneh et al. compared open, laparoscopic and robotic APR and found that MIS was associated with better overall morbidity rates than open; the robotic technique was also associated with lower conversion rates than laparoscopy (34). Our unpublished institutional data suggest that patients robotic APR may be used safely and has comparable perioperative and financial outcomes with LRCS.
The endo-wristed nature of the robotic platform poses a great advantage over laparoscopy because it not only provides better maneuverability during dissection of the tissues surrounding the rectum but also facilitates transection of the rectum via the robotic stapler device, which provided better articulation skills. A widened range of motion and 90° of articulation led to a better angle of transection, fewer stapler firings and decreased cost (35). Yet, the literature lacks data regarding the robotic stapler.
Future directions in robotic surgery
Single-incision laparoscopic surgery (SILS) has been introduced to decrease port-related complications, but technical challenges disrupted the development of SILS, especially in more complex operations where delicate tissue manipulation is needed. Robotic surgery has the ability to overcome such restrictions by increasing articulation, which in turn decreases the risk of instrument collision (36). Combining both surgical concepts may give surgeons the ability to achieve a relatively “scarless” operation with better surgical dynamics.
Following the introduction of SILS, single port systems subsequently led to the development of the transanal MIS technique, which is a derivative of natural orifice transluminal endoscopic surgery. Use of the TME concept via a transanal MIS approach (TaTME) has been associated with comparable surgical outcomes and better oncologic results for low and mid-rectal cancers especially (37-39). The COLOR III randomized trial (40) will provide a better perspective in terms of TaTME for the surgical community. The merits of using a robotic system led surgeons to adapt the robotic platform into TaTME in 2014 for the first time (41), and since then, the robotic TaTME approach has shown feasibility (42). Nonetheless, the bulky arms of da Vinci Xi platform are a drawback in endoluminal surgery. New systems with superior ergonomics in confined spaces have been recently approved including the Flex® Robotic System and Flex® Colorectal (CR) Drive (MedRobotics, Corp. Raynham, MA, USA).
Many solutions have been proposed to decrease rates of anastomotic leaks—one of the most dreadful complications in colorectal surgery where the underlying mechanism is thought to be insufficient tissue perfusion at the anastomosis site. Deciding where to place the anastomotic site mainly depends on active bleeding edge, mesenteric pulsatility and the color of the rectal segment. Lack of tactile feedback in MIS can make this difficult, although using a near-infrared camera on the robotic platform can allow surgeons to visualize the vascular structure of the colon following indocyanine green injection and also help them identify the sentinel lymph node (43).
Although robotic platform offers ample opportunities for further innovations and constitutes a promising frontier in RCS, factors such as increased costs and prolonged operative time are still considered problems that prevent RRCS from being used more routinely. However, the as field of RCS continues to evolve and as the healthcare marketplace becomes more competitive, robotic surgery carries the potential to be the nexus for continued superior clinical outcomes.
Acknowledgments
The tables in this manuscript are reproduced from the article published in Journal of Surgical Oncology. The authors would like to thank Amy Moore for her assistance during the review process of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Eduardo Ma Targarona and Andrea Balla) for the series “Rectal Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.03.12). The series “Rectal Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stevenson AR, Solomon MJ, Lumley JW, et al. ALaCaRT Investigators. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015;314:1356-63. [Crossref] [PubMed]
- Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015;314:1346-55. [Crossref] [PubMed]
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Jayne D, Pigazzi A, Marshall H, et al. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA 2017;318:1569-80. [Crossref] [PubMed]
- Gorgun E, Ozben V, Costedio M, et al. Robotic versus conventional laparoscopic rectal cancer surgery in obese patients. Colorectal Dis 2016;18:1063-71. [Crossref] [PubMed]
- Stulberg JJ, Delaney CP, Neuhauser DV, et al. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA 2010;303:2479-85. [Crossref] [PubMed]
- Guillou PJ, Quirke P, Thorpe H, et al. MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718-26. [Crossref] [PubMed]
- Jayne DG, Guillou PJ, Thorpe HUK MRC CLASICC Trial Group, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 2007;25:3061-8. [Crossref] [PubMed]
- Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 2014;15:767-74. [Crossref] [PubMed]
- van der Pas MH, Haglind E, Cuesta MA, et al. COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Bonjer HJ, Deijen CL, Abis GA, et al. COLOR II Study Group. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;372:1324-32. [Crossref] [PubMed]
- Barnajian M, Pettet D 3rd, Kazi E, et al. Quality of total mesorectal excision and depth of circumferential resection margin in rectal cancer: a matched comparison of the first 20 robotic cases. Colorectal Dis 2014;16:603-9. [Crossref] [PubMed]
- Kim JC, Yu CS, Lim SB, et al. Comparative analysis focusing on surgical and early oncological outcomes of open, laparoscopy-assisted, and robot-assisted approaches in rectal cancer patients. Int J Colorectal Dis 2016;31:1179-87. [Crossref] [PubMed]
- Cho MS, Baek SJ, Hur H, et al. Short and long-term outcomes of robotic versus laparoscopic total mesorectal excision for rectal cancer: a case-matched retrospective study. Medicine (Baltimore) 2015;94:e522 [Crossref] [PubMed]
- Allemann P, Duvoisin C, Di Mare L, et al. Robotic-Assisted Surgery Improves the Quality of Total Mesorectal Excision for Rectal Cancer Compared to Laparoscopy: Results of a Case-Controlled Analysis. World J Surg 2016;40:1010-6. [Crossref] [PubMed]
- Feroci F, Vannucchi A, Bianchi PP, et al. Total mesorectal excision for mid and low rectal cancer: Laparoscopic vs robotic surgery. World J Gastroenterol 2016;22:3602-10. [Crossref] [PubMed]
- Ramji KM, Cleghorn MC, Josse JM, et al. Comparison of clinical and economic outcomes between robotic, laparoscopic, and open rectal cancer surgery: early experience at a tertiary care center. Surg Endosc 2016;30:1337-43. [Crossref] [PubMed]
- Kang J, Yoon KJ, Min BS, et al. The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison--open, laparoscopic, and robotic surgery. Ann Surg 2013;257:95-101. [Crossref] [PubMed]
- Memon S, Heriot AG, Murphy DG, et al. Robotic versus laparoscopic proctectomy for rectal cancer: a meta-analysis. Ann Surg Oncol 2012;19:2095-101. [Crossref] [PubMed]
- Cui Y, Li C, Xu Z, et al. Robot-assisted versus conventional laparoscopic operation in anus-preserving rectal cancer: a meta-analysis. Ther Clin Risk Manag 2017;13:1247-57. [Crossref] [PubMed]
- Kim NK, Kang J. Optimal total mesorectal excision for rectal cancer: The role of robotic surgery from an expert’s view. J Korean Soc Coloproctol 2010;26:377-87. [Crossref] [PubMed]
- Baek JH, Pastor C, Pigazzi A. Robotic and laparoscopic total mesorectal excision for rectal cancer: A case-matched study. Surg Endosc 2011;25:521-5. [Crossref] [PubMed]
- D’Annibale A, Pernazza G, Monsellato I, et al. Total mesorectal excision: A comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc 2013;27:1887-95. [Crossref] [PubMed]
- Yoo BE, Cho JS, Shin JW, et al. Robotic versus laparoscopic intersphincteric resection for low rectal cancer: comparison of the operative, oncological, and functional outcomes. Ann Surg Oncol 2015;22:1219-25. [Crossref] [PubMed]
- Silva-Velazco J, Dietz DW, Stocchi L, et al. Considering Value in Rectal Cancer Surgery: An Analysis of Costs and Outcomes Based on the Open, Laparoscopic, and Robotic Approach for Proctectomy. Ann Surg 2017;265:960-8. [Crossref] [PubMed]
- Morelli L, Guadagni S, Lorenzoni V, et al. Robot-assisted versus laparoscopic rectal resection for cancer in a single surgeon's experience: a cost analysis covering the initial 50 robotic cases with the da Vinci Si. Int J Colorectal Dis 2016;31:1639-48. [Crossref] [PubMed]
- Jiménez-Rodríguez RM, Díaz-Pavón JM, de la Portilla de Juan F, et al. Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis 2013;28:815-21. [Crossref] [PubMed]
- Sng KK, Hara M, Shin JW, et al. The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc 2013;27:3297-307. [Crossref] [PubMed]
- Yamaguchi T, Kinugasa Y, Shiomi A, et al. Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 2015;29:1679-85. [Crossref] [PubMed]
- Bege T, Lelong B, Esterni B, et al. The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution's experience. Ann Surg 2010;251:249-53. [Crossref] [PubMed]
- Kayano H, Okuda J, Tanaka K, et al. Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc 2011;25:2972-9. [Crossref] [PubMed]
- Ito M, Sugito M, Kobayashi A, et al. Influence of learning curve on short-term results after laparoscopic resection for rectal cancer. Surg Endosc 2009;23:403-8. [Crossref] [PubMed]
- Park EJ, Kim CW, Cho MS, et al. Is the learning curve of robotic low anterior resection shorter than laparoscopic low anterior resection for rectal cancer?: a comparative analysis of clinicopathologic outcomes between robotic and laparoscopic surgeries. Medicine (Baltimore) 2014;93:e109 [Crossref] [PubMed]
- Moghadamyeghaneh Z, Phelan M, Smith BR, et al. Outcomes of Open, Laparoscopic, and Robotic Abdominoperineal Resections in Patients with Rectal Cancer. Dis Colon Rectum 2015;58:1123-9. [Crossref] [PubMed]
- Holzmacher JL, Luka S, Aziz M, et al. The Use of Robotic and Laparoscopic Surgical Stapling Devices During Minimally Invasive Colon and Rectal Surgery: A Comparison. J Laparoendosc Adv Surg Tech A 2017;27:151-5. [Crossref] [PubMed]
- Kim SJ, Choi BJ, Lee SC. Overview of single-port laparoscopic surgery for colorectal cancers: past, present, and the future. World J Gastroenterol 2014;20:997-1004. [Crossref] [PubMed]
- Atallah S, Martin-Perez B, Albert M, et al. Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech Coloproctol 2014;18:473-80. [Crossref] [PubMed]
- Lacy AM, Tasende MM, Delgado S, et al. Transanal total mesorectal excision for rectal cancer: outcomes after 140 patients. J Am Coll Surg 2015;221:415-23. [Crossref] [PubMed]
- Tuech JJ, Karoui M, Lelong B, et al. A step toward NOTES total mesorectal excision for rectal cancer: endoscopic transanal proctectomy. Ann Surg 2015;261:228-33. [Crossref] [PubMed]
- Deijen CL, Velthuis S, Tsai A, et al. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc 2016;30:3210-5. [Crossref] [PubMed]
- Atallah S, Nassif G, Polavarapu H, et al. Robotic-assisted transanal surgery for total mesorectal excision (RATS-TME): a description of a novel surgical approach with video demonstration. Tech Coloproctol 2013;17:441-7. [Crossref] [PubMed]
- Atallah S, Martin-Perez B, Parra-Davila E, et al. Robotic transanal surgery for local excision of rectal neoplasia, transanal total mesorectal excision, and repair of complex fistulae: clinical experience with the first 18 cases at a single institution. Tech Coloproctol 2015;19:401-10. [Crossref] [PubMed]
- Buchs N. New trends in robotic colorectal surgery. Adv Robot Autom 2014;3:e117 [Crossref]
Cite this article as: Cengiz TB, Gorgun E. Robotic approach for rectal cancer, is the best approach? Ann Laparosc Endosc Surg 2018;3:28.