
Lymph node mapping with near-infrared fluorescence imaging during robotic surgery for gastric cancer: a pilot study
Introduction
Laparoscopic surgery has emerged as a valid option for treatment of gastric cancer during the last two decades, especially in the East and for patients with early-stage tumors (1). In the Western world, minimally invasive surgery for gastric cancer has not garnered similar popularity and this is mainly due to the lower incidence of early gastric cancer and the complexity of the laparoscopic procedure (2,3).
Gastrectomy with D2 lymphadenectomy, i.e., lymph node dissection along the great vessels, is considered to be technically demanding to perform in laparoscopy (4-6). Robotic surgery has been introduced to overcome the technical difficulties of traditional laparoscopy: magnified 3D imaging and articulating instruments with a greater range of motion can help the surgeon to perform complex surgical steps during radical gastrectomy, such as an accurate lymph node dissection and intracorporeal anastomoses (7-9). Moreover, the daVinci Si robotic platform (Intuitive Surgical Inc., Sunnyvale, CA, USA) is equipped with a near-infrared fluorescence imaging (NIFI) system which allows intraoperative imaging with indocyanine green (ICG) (10). This technology has emerged as a promising intraoperative procedure for lymphatic mapping in gynecological (11), urological (12) and esophageal (13) cancers. In early gastric cancer, NIFI has already been used to identify the sentinel lymph node and thus modulate lymphadenectomy during minimally invasive surgery (14,15).
We hypothesized that robot-assisted NIFI with ICG could be developed for intraoperative lymph node visualization during gastric surgery with the specific aim to help the surgeons to perform a complete D2 lymphadenectomy.
Material and methods
Patients
Fourteen patients diagnosed with gastric adenocarcinoma and scheduled to undergo robotic gastrectomy were enrolled between January and October 2016 at the Center of Oncologic Minimally Invasive Surgery (COMIS), University of Florence, Florence, Italy. All patients underwent preoperative upper digestive endoscopy with gastric biopsy and computed tomography of the abdomen and chest. Patients with history of iodide or seafood allergy and pre- or intraoperative diagnosis of M1 or T4 lesions (i.e., with distant metastases, local invasion of peritoneum, spleen or pancreas), were excluded from the study. None of the patients had received any preoperative radiotherapy and/or chemotherapy. All patients had been thoroughly informed about the study and gave their written consent for the investigation in compliance with the Helsinki Declaration and in accordance with the ethical committee of our University Hospital, Azienda Ospedaliero-Universitaria Careggi (Florence, Italy).
Endoscopic and surgical technique
A 0.2% ICG solution was injected into the submucosa layer with 0.5 mL into the four quadrants around the tumor under endoscopic examination as previously described by Tajima et al. (14) (Figure 1). The dye was injected 1 day before surgery. All patients underwent curative distal gastrectomy with D2 lymph node dissection. The robotic procedures were performed by one surgeon (F.C.) as previously described (16). Intraoperative fluorescence imaging with ICG was carried out with a near-infrared camera system (Firefly Fluorescence Imaging Scope; Intuitive Surgical, Sunnyvale, CA, USA) built into the robotic platform. Lymph nodes which had taken up ICG appeared as green spots emitting clear fluorescence (Figure 2) and were defined as the fluorescent nodes (FNs). The FNs were intraoperatively dissected from the surrounding fatty tissue and pulled out through the assistant 12 mm trocar. The lymph nodes removed with the surgical specimens were isolated from the surrounding tissues on the back table and examined to determine, with the help of the robotic camera if they also exhibited fluorescence. Location and fluorescence status were recorded for all the lymph nodes before they were sent for pathological analysis. Particularly, the dissected lymph nodes were grouped into five gastric lymphatic basins along the main arteries as previously described by Kinami et al. (17): left gastric artery (l-GA), right gastric artery (r-GA), right gastroepiploic artery (r-GEA), left gastroepiploic artery (l-GEA) and posterior gastric artery (p-GA). The l-GA area consisted of lymph node stations 1, 2, 3, 7 and 9. The r-GA area consisted of stations 5, 8a, 8p and 12a. The r-GEA consisted of stations 4d and 6. The l-GEA consisted of stations 4sa and 4sb. The pGA consisted of stations 10, 11p and 11d.
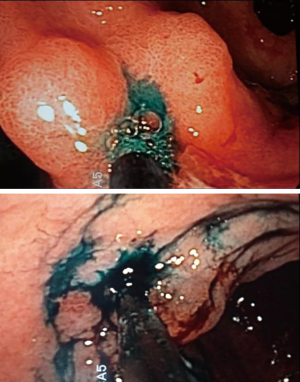
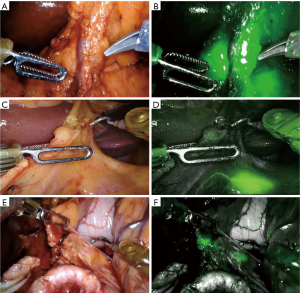
Pathological analysis
Clinicopathological findings such as tumor location, histotype, tumor differentiation, Lauren classification, depth of invasion, lymph node metastasis and stage distribution were reviewed according to the Japanese gastric carcinoma classification (18). All dissected lymph nodes were examined histologically one slice per node and stained with H&E.
Results
Fourteen patients (8 males and 6 females) were enrolled in the study. Their median age was 76 years (range, 49–81 years) and mean BMI was 25.2 kg/m2. Patients’ clinicopathological characteristics are shown in Table 1. There were no patients with complications or adverse events after ICG injection in this pilot study. All patients underwent a robotic distal gastrectomy with standardized D2 lymphadenectomy. In situ detection of NIFI at the site of the gastric tumor was achieved in all cases. The mean total number of retrieved and examined lymph nodes was 43.3 (range, 27–78) (Table 2). The mean number of FNs was 19.4 (range, 1–36) (Table 2). Dye diffusion to lymph nodes was observed in all the patients (Table 2). No ICG staining was observed in those lymph nodes (namely station 2, 4sa, 10 and 11 d) for which dissection is not suggested during distal gastrectomy. Patient 11 had only one FN retrieved, and this was most likely due to the pathological finding of massive infiltration of the submucosa (linitis plastic) that might have occluded the lymphatic vessels and prevented ICG diffusion. The histopathological analysis indicated that 7 patients had metastatic lymph nodes: all the metastatic lymph nodes were fluorescent in 3 patients, they were non-fluorescent in 3 and both fluorescent and non-fluorescent in 1 (Table 2). The distribution of lymph nodes and their metastatic/fluorescent status within the five lymphatic basins are shown in Table 3. It is of interest that in patients n. 1 and 6, the metastatic non-fluorescent lymph nodes were found within the lymphatic basins which were marked by at least one FN (Table 3).
Table 1
| Characteristics | Patients (N=14) |
|---|---|
| Gender (male/female) | 8/6 |
| Age (year) [median, range] | 76 [49–81] |
| BMI (kg/m2) [median, range] | 25.2 [23–30] |
| Tumor location (%) | |
| Middle third | 7 (50.0) |
| Lower third | 7 (50.0) |
| Lauren classification (%) | |
| Intestinal | 8 (57.2) |
| Diffuse | 1 (7.1) |
| Mixed | 5 (35.7) |
| Tumor differentiation (%) | |
| Well differentiated | 4 (28.6) |
| Moderately differentiated | 7 (50.0) |
| Poorly differentiated | 3 (21.4) |
| Tumor size (cm) (mean ± SD) | 3.8±2.1 |
| Stage distribution (%) | |
| I | 6 (42.9) |
| II | 5 (35.7) |
| III | 3 (21.4) |
Table 2
| Pt n. | TNM | Total examined LNs | Fluorescent LNs (%) | Fluorescent metastatic LNs | Non-fluorescent metastatic LNs |
|---|---|---|---|---|---|
| 1 | T1N2 | 42 | 35 (83.3) | 0 | 3 |
| 2 | T1N0 | 60 | 34 (56.6) | 0 | 0 |
| 3 | T2N1 | 47 | 36 (76.5) | 1 | 0 |
| 4 | T2N0 | 49 | 34 (69.3) | 0 | 0 |
| 5 | T2N0 | 44 | 5 (11.3) | 0 | 0 |
| 6 | T3N1 | 27 | 5 (18.5) | 0 | 1 |
| 7 | T2N0 | 49 | 23 (46.9) | 0 | 0 |
| 8 | T3N3 | 78 | 31 (39.7) | 7 | 7 |
| 9 | T1N0 | 53 | 8 (15.0) | 0 | 0 |
| 10 | T1N0 | 34 | 12 (35.2) | 0 | 0 |
| 11 | T3N3 | 27 | 1 (3.7) | 0 | 7 |
| 12 | T2N2 | 45 | 7 (18.4) | 5 | 0 |
| 13 | T2N2 | 63 | 36 (57.1) | 4 | 0 |
| 14 | T2N0 | 27 | 5 (18.5) | 0 | 0 |
| Overall (mean ± ESM) | 43.3±3.7 | 19.4±3.8 | 1.2±0.6 | 1.2±0.6 |
Table 3
| Pt n. | Tumor location | Tumor size (cm) | Left GA | Left GEA | Posterior GA | Right GEA | Right GA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluorescent | Non | Fluorescent | Non | Fluorescent | Non | Fluorescent | Non | Fluorescent | Non | |||||||
| 1 | Middle | 2 | 6 | 2 | 0 | 0 | 9 | 1 | 18 | 1 | 2 | 3 (3) | ||||
| 2 | Middle | 2.5 | 15 | 6 | 0 | 0 | 0 | 0 | 3 | 19 | 16 | 1 | ||||
| 3 | Lower | 4 | 18 | 0 | 0 | 1 | 8 | 0 | 7 (1) | 2 | 3 | 8 | ||||
| 4 | Lower | 4 | 9 | 7 | 0 | 0 | 8 | 0 | 5 | 5 | 12 | 3 | ||||
| 5 | Lower | 2.5 | 3 | 7 | 0 | 0 | 0 | 5 | 2 | 12 | 0 | 15 | ||||
| 6 | Lower | 5.5 | 2 | 10 | 0 | 0 | 0 | 2 | 2 | 9 | 1 | 1 (1) | ||||
| 7 | Middle | 4 | 11 | 10 | 0 | 0 | 2 | 1 | 5 | 4 | 5 | 11 | ||||
| 8 | Lower | 8 | 7 | 5 | 0 | 5 | 0 | 8 | 13 (3) | 6 (3) | 11 (4) | 23 (4) | ||||
| 9 | Middle | 2 | 1 | 24 | 0 | 0 | 0 | 4 | 7 | 6 | 0 | 11 | ||||
| 10 | Lower | 5 | 5 | 6 | 0 | 0 | 0 | 0 | 7 | 10 | 0 | 6 | ||||
| 11 | Middle | 7 | 0 | 18 (5) | 0 | 2 | 0 | 1 (1) | 5 | 1 (1) | 0 | 0 | ||||
| 12 | Lower | 2.5 | 0 | 16 | 0 | 0 | 0 | 5 | 5 (4) | 8 | 2 (1) | 9 | ||||
| 13 | Middle | 1 | 12 | 0 | 0 | 0 | 5 (1) | 2 | 11 (3) | 6 | 8 | 19 | ||||
| 14 | Middle | 2.5 | 0 | 1 | 0 | 0 | 0 | 0 | 5 | 1 | 0 | 20 | ||||
GA, gastric artery; GEA, gastroepiploic artery; (): number of metastatic lymph nodes.
Discussion
Adequate lymph node sampling during radical gastrectomy is crucial for proper staging of patients with gastric cancer. In this pilot study, we evaluated the feasibility and safety of a novel application of NIFI with ICG for real-time intraoperative mapping of lymph nodes during robotic surgery for gastric cancer. ICG is a diagnostic reagent that has excitation and fluorescence wave-lengths in the near-infrared range. Nimura et al. (19) have reported that lymphatic vessels and lymph nodes containing ICG particle can be easily distinguished from surrounding fatty tissue using infrared ray technology system. In addition, ICG deposition and fluorescence imaging are characteristically found for prolonged periods of time in the lymph nodes (>3 days).
An intraoperative imaging system based on NIFI with ICG has already been proposed for sentinel lymph node mapping in early gastric cancer (14,15,20,21). To our knowledge, this is the third pilot trial designed to visualize intraoperatively the lymphatic pathways draining gastric tumors using NIFI with ICG without the purpose of sentinel lymph node retrieval. Herrera-Almario et al. (22) used this technique in 29 patients who had undergone robotic resection for gastric adenocarcinoma: they demonstrated the safety and feasibility of the procedure and stated that it could be a valuable adjunct for overall lymph node retrieval. They found that a mean of 29 lymph nodes were examined and, in all cases, at least 5 lymph nodes were seen along the main nodal compartments. Lan et al. (23) compared 14 and 65 patients who underwent robotic gastrectomy with or without ICG fluorescence, respectively. They did not find any significant differences in the total number of lymph nodes retrieved in the two groups, but all the metastatic lymph nodes were found in the lymph node stations which showed fluorescence signals. Our preliminary study confirmed the feasibility and safety of the procedure and interestingly, we found a higher number of lymph nodes in the surgical specimens than the other two previously published studies, with a mean of 43.3. We hypothesize that this advantage was due to the different method of dye injection. In the study by Herrera-Almario (22), ICG was injected intraoperatively into the subserosa around the tumor in all their 31 patients whereas the same method was adopted in 9 out of the 14 patients investigated by Lan et al. (23). It is most likely that, with subserosal injection, the dye did not have enough time to spread into the lymphatic vessels and deposit in all draining lymph nodes. Furthermore, the endoscopic submucosal injection of ICG, unlike the subserosal approach, avoids any potential lymphatic disruption that can occur with intraoperative dissection and permits direct visualization of the lesion at the time of surgery. Other important limitations of subserosal injection are the potential intraoperative leakage of ICG with spoiling of the near infrared view and the difficulty to identify tumor location from the outside of the stomach without intraoperative localization of the tumor, especially in the cases of early gastric cancer.
We found that the most important factor in determining the image quality during intraoperative fluorescence imaging is the dosage of ICG injection. In our very first cases, not considered in the present study, we injected 1 mL of 0.2% ICG solution into the four quadrants around the tumor but the patients had a dissemination of the fluorescence signal in the omentum, mesocolon and pancreatic surface. We obtained a better quality of fluorescence imaging by reducing the ICG dosage to 0.5 mL.
Importantly, the mean value of retrieved and examined lymph nodes in the present study was even higher than that we found in a group of patients who were operated on by robotic distal gastrectomy without intraoperative ICG-NIFI (43.3 vs. 39.1, respectively) between June 2014 and September 2015 (16).
We also found that metastatic disease was present in FNs in 4 out of our 7 patients classified as N+, whereas metastatic non-fluorescent lymph nodes were found within lymphatic basins with fluorescence signals in 2 other cases. These findings confirmed those reported by Lan et al. (23) and suggest that intraoperative lymphatic mapping with ICG fluorescence has the potential to improve the likelihood of an adequate lymphadenectomy by identifying those lymphatic basins that most likely contain metastatic disease.
In conclusion, we recognize that this pilot trial includes only a small patient sample and that future trials are needed to truly determine the impact of intraoperative lymphatic mapping with ICG fluorescence on gastric cancer surgery. However, our preliminary results suggest that this technique is feasible and can help the surgeon to have a real-time visual reference of the lymph nodes during dissection along the main gastric vessels, thus adding a potentially valuable adjunct to perform a complete D2 lymphadenectomy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.03.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). All patients had been thoroughly informed about the study and gave their written consent for the investigation in compliance with the Helsinki Declaration and in accordance with the ethical committee of our University Hospital, Azienda Ospedaliero-Universitaria Careggi (Florence, Italy).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koeda K, Nishizuka S, Wakabayashi G. Minimally invasive surgery for gastric cancer: the future standard of care. World J Surg 2011;35:1469-77. [Crossref] [PubMed]
- Strong VE, Devaud N, Karpeh M. The role of laparoscopy for gastric surgery in the West. Gastric Cancer 2009;12:127-31. [Crossref] [PubMed]
- Yamamoto M, Rashid OM, Wong J. Surgical management of gastric cancer: the East vs. West perspective. J Gastrointest Oncol 2015;6:79-88. [PubMed]
- Coburn NG. Lymph nodes and gastric cancer. J Surg Oncol 2009;99:199-206. [Crossref] [PubMed]
- Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol 2005;11:7508-11. [Crossref] [PubMed]
- Zou ZH, Zhao LY, Mou TY, et al. Laparoscopic vs open D2 gastrectomy for locally advanced gastric cancer: a meta-analysis. World J Gastroenterol 2014;20:16750-64. [Crossref] [PubMed]
- Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg 2004;239:14-21. [Crossref] [PubMed]
- Diana M, Marescaux J. Robotic surgery. Br J Surg 2015;102:e15-28. [Crossref] [PubMed]
- Obama K, Sakai Y. Current status of robotic gastrectomy for gastric cancer. Surg Today 2016;46:528-34. [Crossref] [PubMed]
- Daskalaki D, Aguilera F, Patton K, et al. Fluorescence in robotic surgery. J Surg Oncol 2015;112:250-6. [Crossref] [PubMed]
- Kimmig R, Aktas B, Buderath P, et al. Intraoperative navigation in robotically assisted compartmental surgery of uterine cancer by visualisation of embryologically derived lymphatic networks with indocyanine-green (ICG). J Surg Oncol 2016;113:554-9. [Crossref] [PubMed]
- Manny TB, Hemal AK. Fluorescence-enhanced robotic radical cystectomy using unconjugated indocyanine green for pelvic lymphangiography, tumor marking, and mesenteric angiography: the initial clinical experience. Urology 2014;83:824-9. [Crossref] [PubMed]
- Hachey KJ, Gilmore DM, Armstrong KW, et al. Safety and feasibility of near-infrared image-guided lymphatic mapping of regional lymph nodes in esophageal cancer. J Thorac Cardiovasc Surg 2016;152:546-54. [Crossref] [PubMed]
- Tajima Y, Murakami M, Yamazaki K, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer. Ann Surg Oncol 2010;17:1787-93. [Crossref] [PubMed]
- Tummers QR, Boogerd LS, de Steur WO, et al. Near-infrared fluorescence sentinel lymph node detection in gastric cancer: A pilot study. World J Gastroenterol 2016;22:3644-51. [Crossref] [PubMed]
- Cianchi F, Indennitate G, Trallori G, et al. Robotic vs laparoscopic distal gastrectomy with D2 lymphadenectomy for gastric cancer: a retrospective comparative mono-institutional study. BMC Surg 2016;16:65. [Crossref] [PubMed]
- Kinami S, Fujimura T, Ojima E, et al. PTD classification: proposal for a new classification of gastric cancer location based on physiological lymphatic flow. Int J Clin Oncol 2008;13:320-9. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, 2nd English ed. Gastric Cancer 1998;1:10-24. [Crossref] [PubMed]
- Nimura H, Narimiya N, Mitsumori N, et al. Infrared ray electronic endoscopy combined with indocyanine green injection for detection of sentinel nodes of patients with gastric cancer. Br J Surg 2004;91:575-9. [Crossref] [PubMed]
- Takahashi N, Nimura H, Fujita T, et al. Laparoscopic sentinel node navigation surgery for early gastric cancer: a prospective multicenter trial. Langenbecks Arch Surg 2017;402:27-32. [Crossref] [PubMed]
- Kinami S, Oonishi T, Fujita J, et al. Optimal settings and accuracy of indocyanine green fluorescence imaging for sentinel node biopsy in early gastric cancer. Oncol Lett 2016;11:4055-62. [Crossref] [PubMed]
- Herrera-Almario G, Patane M, Sarkaria I, et al. Initial report of near-infrared fluorescence imaging as an intraoperative adjunct for lymph node harvesting during robot-assisted laparoscopic gastrectomy. J Surg Oncol 2016;113:768-70. [Crossref] [PubMed]
- Lan YT, Huang KH, Chen PH, et al. A pilot study of lymph node mapping with indocyanine green in robotic gastrectomy for gastric cancer. SAGE Open Med 2017;5:2050312117727444 [Crossref] [PubMed]
Cite this article as: Cianchi F, Indennitate G, Trallori G, Paoli B, Ortolani M, Taddei A, Lami G, Foppa C, Badii B, Novelli L, Skalamera I, Montanelli P, Coratti F, Perigli G, Staderini F. Lymph node mapping with near-infrared fluorescence imaging during robotic surgery for gastric cancer: a pilot study. Ann Laparosc Endosc Surg 2018;3:31.


