
Transanal total mesorectal excision compared to laparoscopic TME for mid and low rectal cancer—current evidence
Background
Transanal total mesorectal excision (TaTME) is the potential answer to refractory challenges in the surgical resection of mid and low rectal cancer. Since the first reports of this approach by its pioneers in 2010, the technique has gained wide attention in the surgical community (1,2). The technique facilitates dissection of the very distal rectum and mesorectum providing excellent view of the anatomy in the deep pelvis. Especially difficult cases (e.g., obese, male patients with bulky distal tumors) seem to benefit from the transanal approach. Nevertheless, no randomized evidence is present to support any benefits. Also, in systematic reviews as well as reported in registries the benefits of TaTME compared to LaTME seem modest and long-term oncological outcome is awaited (3-5). Current problems in TME surgery are short-term morbidity including anastomotic leakage, conversions to open surgery in 10–25%, unintended end colostomies, poor specimen quality, circumferential resection margin (CRM) involvement, distal margins rates and local recurrences (LR).
Laparoscopic TME (LaTME) surgery is associated with substantial short-term morbidity of 30–40%. This includes anastomotic leakage rates around 8% (6,7). The conversion rate in LaTME is still above 10% as reported in recent trials and even with robotic surgery this percentage remains between 10% and 20%, especially in obese patients (8-11). Conversion to open surgery occurs due to difficult dissection and is associated with higher morbidity and worse oncological outcome (12-15).
Currently an increase focus is seen towards sphincter preserving therapy (16,17). The open intersphincteric resection and transanal dissection creates the possibility of saving the sphincter avoiding end colostomies, but LaTME still results in a relatively high rate of APR, which has negative impact on quality of life. Transanal minimal invasive access with high-quality images creates the potential to achieve a higher rate of sphincter saving procedures. Nevertheless, data regarding unintended AP resections are scarce and only within a randomized comparison it will be possible to evaluate this aspect. High-quality surgery with respect to the embryological avascular planes aims to achieve an intact mesorectal envelope and offers good local control, especially with neoadjuvant radiotherapy if indicated (18,19). An involved CRM remains a concern since this is a substantial risk factor for LRs. Laparoscopic surgery with an intent for TME result in an involved CRM of 17% as is shown in national registries (20). Potentially the mesorectum is not totally removed in TME surgery as shown by the presence of residual mesorectum in 40% upon evaluation by MRI 6 months after surgery in 46.9% (21).
The TaTME technique could improve outcomes for patients with mid and low rectal cancer overcoming the limitations of dissection the angulated rectum deep within the pelvis (1,12). Especially the known difficult LaTME resection will probably benefit from the TaTME approach such as male sex, low tumor, high body mass index (BMI) and bulky or anteriorly situated tumours (22). In these situations, deep mesorectal dissection, safe resection margins (both distal and circumferential) and safe stapled transection, without the need for multiple firings, may not be achieved requiring conversion to open surgery (12,20). Furthermore, in (ultra)low anterior resections for tumours situated close to but not grown into the pelvic floor, the rate of end-colostomy (APR) for technical considerations outstands the rate in which it is an oncological necessity (16). TaTME may overcome these challenges by improved visualization and ergonomics. By enabling a more precise distal dissection in the embryological planes, theoretically the autonomic nerves can be preserved possibly leading to improved functional outcomes (23,24).
In this review we focus to current evidence of laparoscopic rectal surgery and TaTME with respect to morbidity including anastomotic leakage, conversion, colostomy, involved resection margins and LRs.
Methods
We performed a PubMed search with a similar syntax as recently published to identify studies, published since January 2005, reporting on outcomes of TaTME (4). The final search was performed at 25 January 2018 with the following syntax:
((((excision*[tiab] OR resection*[tiab] OR TME[tiab] OR TaTME[tiab] OR TAMIS[tiab] OR NOTES[tiab] OR proctectom*[tiab]) AND (transanal*[tiab] OR trans-anal*[tiab])) OR ((excision*[ot] OR resection*[ot] OR TME[ot] OR TaTME[ot] OR TAMIS[ot] OR NOTES[ot] OR proctectom*[ot]) AND (transanal*[ot] OR trans-anal*[ot]))) AND (((("Neoplasms"[Mesh] OR neoplas*[tw] OR tumor*[tw] OR tumour*[tw] OR cancer*[tw] OR malignan*[tw] OR oncolog*[tw] OR carcinom*[tw] OR adenocarcinom*[tw]) AND ("Rectum"[Mesh] OR rectum[tiab] OR rectal[tiab] OR colorect*[tiab] OR mesorect*[tiab])) AND ("surgery"[Subheading] OR surgery[tiab] OR surgical[tiab] OR operati*[tiab])) OR (“Rectal Neoplasms/surgery”[Mesh:noexp]))).
For this paper, case-series, cohorts and comparative studies, with a minimum of 15 patients that underwent Transanal TME for rectal cancer were included. The transanal approach had to be an endoscopic technique, therefore papers on open transanal approach (TaTa) were excluded. Moreover, animal and cadaver studies were not included. Language in which the cohorts were reported was restricted languages with the Latin alphabet.
The international registry of TaTME was isolated from the other retrieved series on TaTME, because of its different design and the rather large proportion of indications other that rectal cancer such as IBD, completion proctectomy and Hartmann reversal (25).
In addition, to provide the most recent evidence from randomized clinical trials regarding LaTME, data from the laparoscopic arms of large, n≥200, multi-center RCT’s published after 01-01-2015 were isolated and recorded.
Primary endpoints were type of surgery, morbidity, anastomotic leakage, defined as (partial) dehiscence, intraoperative complication as urethral injury, intraoperative bleeding, stoma-rate, conversion, clinicopathological parameters as quality of the mesorectum, CRM and distal margin involvement and long-term oncological outcome as LRs.
Statistical analysis
Because a minority reported comparative data on TaTME and LaTME, no direct comparative meta-analysis could be performed. Instead, for the retrieved laparoscopic and TaTME studies, a separate weighted average was provided for the retrieved baseline characteristics and outcomes. The calculated weighted percentages and crude data (events and adjusted total population per outcome) of the LaTME and retrieved TaTME series, as well as the TaTME registry are presented in tables.
For the primary endpoints, if possible, a separate weighted average of the proportions was determined by means of the generic inverse-variance method. This is a method for aggregating multiple effect sizes to minimize the variance of the weighted average, giving more weight to the effect of large studies than to small ones. Analyses were performed with the inverse-variance method, using a random-effects model. Heterogeneity was assessed by use of the I2 statistic. The software used for statistical analysis was R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The PubMed search on TaTME resulted in 1,093 papers. Selection by title/abstract excluded 1,058 studies which resulted in 35 papers for full text analysis. Of those, nine papers were excluded for N≤15 (26-34). Other reasons of exclusion were the use of another technique or non-availability of full-text for two other articles (35,36). Eventually, 24 papers on TaTME were included in this paper, of which overlap existed by 2 papers from the same clinic (37,38). Since the latter paper focused on pathological outcomes, from this paper only the pathological data were extracted; quality of mesorectum, CRM and DRM positivity (38). Because of its unique design the TaTME registry was not pooled with the other series in the meta-analysis (25). See Figure 1 flowchart and Table S1 search results.
The selection of the open/robotic versus LaTME multicenter RCT’s with an arm of N≥200 resulted in the ROLARR, COLOR II, ALaCaRT and ACOSOG Z6051 (8,9,39-41).
TaTME series and registry
The 23 included TaTME cohorts varied in design and inclusion criteria and reported clinical and pathological outcomes of 1,107 patients, online: http://ales.amegroups.com/public/system/ales/supp-ales.2018.04.02-2.pdf TaTME (37,38,42-62). Fourteen single center series (37,43-47,51,53-55,57-60), of which one published 2 papers (37,38), 7 dual or multicenter (42,48,50,53,56,61,62) and one paper of an implementation pathway (49) were included. One of the single center papers included solely advanced or recurrent low rectal cancers (59). Furthermore, for the pathological data of the largest single center experience, Hospital Clinic, we extracted the data of their latest paper on pathological endpoints (38). The indication for TaTME was merely rectal cancer varying from <5 to 15 centimeter from the anal verge, however some included a few benign cases. Due to heterogeneous inclusion criteria regarding intent for continuity; pooling for APR or LAR rate could not be performed (Table 1). Baseline characteristics, Surgical and postoperative outcomes of the TaTME series can be found in online: http://ales.amegroups.com/public/system/ales/supp-ales.2018.04.02-2.pdf TaTME.
Table 1
| Outcome | TaTME | LaTME | Registry |
|---|---|---|---|
| Baseline | |||
| Population (N) | 1,107 | 1,411 | 1,594 |
| Male (sex) | 65.3% (710/1,088) | 65% (923/1,411) | 68% (1,080/1,594) |
| Age (years, RoM) | 63.7 (55.0–70.0) | 64.7 (57.7–66.8) | 63.7 |
| BMI (RoM) | 26.2 (24.2–29.5) | 26.3 (26.1–27.0) | 26.3 |
| Neoadj treatment | 65.3% (680/1,041) | 61.9% (874/1,411) | 56% (895/1,594) |
| Surgery | |||
| Colostomy (definite) | 7.4% (75/1,007) | 27.0% (378/1,398) | 12% (211/1,755)* |
| Conversion | 2.0% (22/1,083) | 13.7% (192/1,403) | 5.6% (90/1,594) |
| Duration of surgery (min, RoM) | 249.1 (166–368.6) | 242.9 (210.0–266.0) | 252 (30.0–733.0) |
| Intraoperative complications | 4.2% (36/865) | 12.1% (141/1,164) | 30.6% (487/1,594) |
| Intraoperative bleeding | 1.7% (10/585) | 3.5% (41/1,164) | 4.2% (67/1,594) |
| Urethral injury | 0.7% (5/694) | NR | 0.8% (12/1,594) |
| Pathology | |||
| Mesorectal integrity | |||
| Complete | 86.2% (871/1,010) | 81.5% (1,139/1,398) | 85.5% (1,193/1,540)** |
| Partial | 12.6% (111/1,010) | 11.9% (166/1,398) | 10.8% (150/1,540)** |
| Incomplete | 2.8% (25/1,010) | 4.1% (58/1,398) | 3.4% (47/1,540)** |
| CRM positive | 4.6% (51/1,118) | 7.9% (102/1,290) | 4.1% (60/1,451) |
| DRM positive | 0.7% (7/1,013) | 1.0% (7/702) | 0.7% (10/1,445) |
| Postoperative outcomes | |||
| Short-term morbidity (30 days) | 31.7% (317/1,000) | 39.6% (462/1,167) | 34.8% (555/1,594) |
| Anastomotic leakage (30 days) *** | 6.9% (67/975) | 8.0% (88/1,104) | 7.8% (124/1,594) |
| Length of stay (days, RoM) | 7.5 (4.0–4.0) | 7.9 (7.3–8.2) | 8 (2.0–94.0) |
| Mortality (30 days) | 0.6% (7/110) | 0.9% (13/1,407) | 0.6% (9/1,594) |
*, included 161 APR; **, minus 54 benign; ***, defined as dehiscence. RoM, range of reported means or medians; DRM, distal resection margin; CRM, circumferential resection margin; BMI, body mass index; TaTME, transanal total mesorectal excision; LaTME, laparoscopic TME.
The TaTME registry encompasses 1,594 patients who received an anastomosis after TaTME, of which 1,540 procedures were for rectal cancer, and in the appendix an additional 161 abdominoperineal resections (APR) were reported (25).
LaTME
The LaTME arms of the before mentioned RCT’s covered short-term and pathological outcomes of 1,411 patients (8,9,39-41). Only the COLOR II trial reported the long-term outcomes: 5% LRs after 3 years (39). Patient demographics were comparable, but Fleshman et al. reported an neo-adjuvant therapy rate of 98% which was rather high compared with the 46–59% rate reported by the other trials, see Table S2 LaTME (41).
The 4 studies reported an APR percentage of 11–29%, and had a weighted colostomy rate of 27% (Table 1). Conversion to open surgery was done in 13.7% and LaTME resulted in 8% anastomotic leakage. The retrieved crude data can be found in Table S2 LaTME.
Short-term morbidity and anastomotic leakage
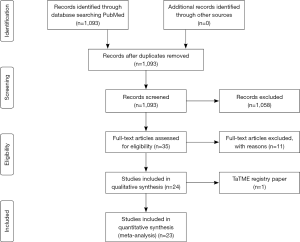
The reported short-term morbidity (30-day) of the retrieved TaTME series varies between 8.7% and 52% with a weighted average of 31.5% versus an average of 39.6% short-term morbidity in laparoscopy (Table 1). In the meta-analysis of short-term morbidity, an average rate of 0.32 (95% CI, 0.28–0.36, I2=25.8%) of TaTME versus 0.39 (95% CI, 0.33–0.46, I2=80.6%) laparoscopy was calculated, but with serious risk at heterogeneity for LaTME. See Figure 2A,B.
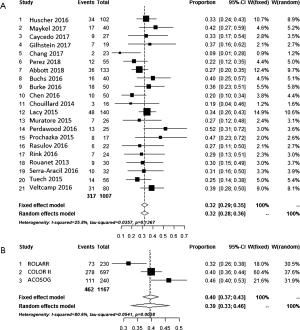
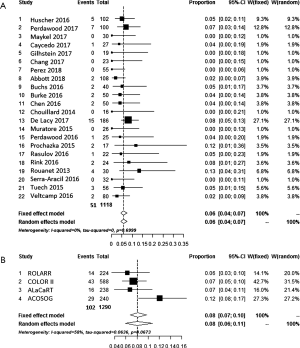
The incidence of anastomotic leakage, defined as (partial dehiscence) was more or less equal for weighted average of laparoscopic (8.0%), TaTME (6.9%) and the Registry (7.8%) (Table 1). Interestingly, anastomotic failure, including pelvic abscess, fistula and sinus reached 15.8% if the 30-day was extended to 3 months (25). The meta-analysis of the weighted average of the proportions can be found in Figure 3A,B.
For TaTME specific intraoperative complications such as pelvic (sidewall or prescaral) bleeding and urethral injury, 10 (1.7%) and 5 (0.7%) cases were reported. The international TaTME registry report 4.2% intraoperative bleeding and 0.8% (n=12) urethral injuries (Table 1). The 30.6% intraoperative complication rate included technical aspects, such as difficulties with the transanal platform (25).
Conversion
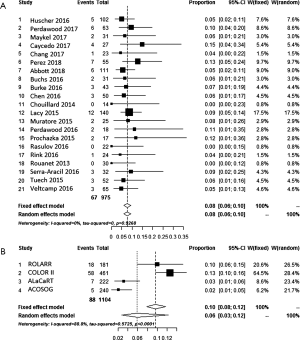
Conversion to open surgery is encountered less frequently in TaTME, 2.0% versus 13.7%. The registry reports an intermediate conversion rate of 5.6% (Table 1). Meta-analysis by inverse variance with a random effects model resulted in a 0.03 (95% CI, 0.02–0.05) rate of conversion in TaTME with low risk of heterogeneity (I2=0%), see Figure 4A. In LaTME this was 0.13 (95% CI, 0.09–0.16, I2=69.6%) with risk of heterogeneity, see Figure 4B.
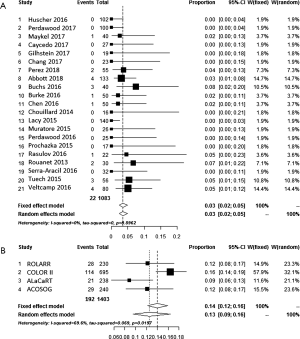
Colostomy
The four RCT’s of LaTME encompassed an average of 20% APR for TME surgery, with a definitive stoma rate of 27% (Tables 1,S2). The retrieved TME series varied in inclusion criteria for APR’s, intersphincteric TME and low anterior resections. Therefore, an average APR-rate could not be calculated, but the reported definitive stoma rate was 7.6%. The registry paper focused on anastomotic leakage and excluded APR’s. However, the supplement stated 161 registered APR’s leading to a 12% definitive stoma rate in which also total proctocolectomies or completion proctectomies for benign indications were included (25).
Irradicality
The integrity of the mesorectum, defined by Quirke, was complete in 81.5% in laparoscopy and 86.2% in TaTME (Table 1). Distal resection margin positivity was 1.0% and 0.7% for LaTME and TaTME respectively. The weighted rate of a positive CRM, was 7.9% versus 4.6% in the LaTME and TaTME groups respectively (Table 1). For the pathological outcomes of the latest paper from the group of Lacy et al. was used (38).
Meta-analysis of complete mesorectum and CRM+ for both approaches are shown in Figure 5A,B and Figure 6A,B. The percentage of complete is 86% vs. 80% in the transanal versus pure laparoscopic approach, but with a high I2 of 90.8% in LaTME. The weighted average of the proportions shows a positive CRM of 0.08 (95% CI, of laparoscopic 0.06–0.11, I2=58%) in laparoscopy compared to 0.06 in TaTME (95% CI, 0.04–0.07, I2=0).
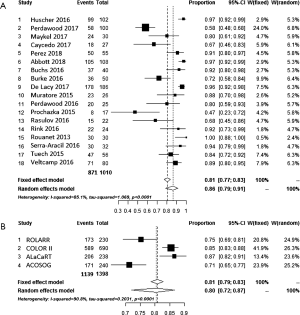
LR
LR rate was reported 5% the 3-year follow-up paper in COLOR II-trial (39). None of the other LaTME trials reported 3-year follow-up yet. Neither the TaTME papers reported 3 years follow-up with LR percentages. However, an incidence of 17 LRs was reported, but (37,46,51,59,61,62). Of these 17 LRs, 5 were reported by Rouanet et al. who included locally advanced rectal cancer or LRs in his TaTME-series (59).
Discussion
This is the latest systematic review of all the cohorts larger than 15 patients, describing the short-term results of TaTME. Because the comparison with LaTME is most important in evaluating the potential benefits of TaTME and comparative prospective clinical trial are still lacking we have added the result of the 4 latest randomized trials evaluating LaTME. TaTME compared to LaTME is beneficial in terms of conversion rate and clinico-pathological outcomes, morbidity and anastomotic leakage seems comparable. End colostomy (APR) rate and LRs could was not reliably reported by the retrieved studies, due to design and lack of long-term follow-up (37,38,42-62). Before widespread implementation can be justified, careful evaluation is warranted because morbidity has not decreased, and the rate of LR is still a concern and needs thorough evaluation in an RCT with quality control of surgery and data (63).
The short-term overall morbidity in TaTME was 31.5% which is beneficial compared to the registry data of 34.8% and pooled LaTME of 39.6%. This 31.5% rate of the TaTME cohorts must be interpreted with caution since morbidity was not reported in a standardized way such as the Clavien-Dindo classification. A concern in (laparoscopic) rectal cancer surgery remains anastomotic leakage which was 8% in the latest laparoscopic RCT data. It was imposed that leakage could be decreased by TaTME as a consequence of a new way of making an anastomosis without the need for cross stapling leaving dog-ears which are prone to ischemia (25,64). However, the current data do not suggest a decrease with a reported 30-day leak rate 7%. The open rectal stump which results after the dissection needs to be fused with the descending colon to create the anastomosis. This can be performed either by a hand sewn colo-anal anastomosis for very low anastomosis or by a stapled colo-rectal anastomosis with a circular stapling technique with the aid of a second purse string to close up the open rectal stump, which is described in detail in the 2016 paper in Techniques of coloproctology (64). TaTME potentially leads to more bacterial load as showed by Velthuis et al. and needs further investigation of the rate of anastomotic failure or pelvic abscess (65). The registry reported an anastomotic failure rate of 15.7% when late complications of the anastomosis such as fistula, chronic abscess or presacral sinuses were included (25). These numbers are comparable to the anastomotic leak rate, defined as not only early dehiscence but include presacral abscess or sinus, which builds up to 20% beyond the 30-day cut-off (6).
This paper shows a promising conversion rate of 2% in TaTME in the cohort series compared to 5.6% in the TaTME registry compared to 12.2–16.6% for LaTME as reported in the ROLARR and laparoscopic arms of the COLOR II, ALaCaRT and ACSOG-Z6501 trials (8,9,40,41). The main reasons for conversion to open surgery are extensive adhesions, intra-operative complications such as major bleeding, the inability to make progress in sharp dissection of the mesorectal envelope or to achieve a clear distal margin below the tumor (12). Risk-factors that contribute to difficulty are male sex, high BMI, visceral obesity, narrow pelvis, bulky tumours or more advanced stage of the tumours and these patients might be candidates for a down to up approach of mesorectal excision (10,11,66).
The average 20% APR-rate in LaTME surgery for rectal cancer as stated before impacts quality of life. This extensive procedure with resection of the sphincter complex is not always required from oncological point of view, which is mostly the risk of CRM+ due to ingrowth in the sphincter complex or levator ani, but is also performed for technical reasons such as the inability to get a satisfactory distal margin intraoperatively (16). Definitive colostomy-rate is even higher, adding some sphincter sparing Hartmann procedures without restoration of bowel continuity. TaTME enables lower sphincter saving, or intersphincteric, dissections with a colo-anal anastomosis in selected cases (16). Unintended APR rate resulting in end colostomy was an endpoint which could not be evaluated. The RCT series did not report the incidence of planned versus unplanned; one study reported an end colostomy rate of 79.7% in LaTME for the low rectum (0–5 cm from the AV) (9).
The CRM positivity, an important predictor of LR, was found to be less frequently involved in TaTME (67). This can be contributed by improved visualization of the surgical plane and improved ergonomics in the dissection of especially the lower (meso)rectum (1,2).
The integrity of the mesorectal envelope surrogates surgical quality by pursuing a smooth specimen which is correlated to LR (68). This review shows that TaTME results in better mesorectal integrity for TaTME as shown in Table 1. Positive distal resection margins are rarely encountered since the tumor can be directly visualized by the transanal endoscopic view (4). Furthermore, in case of a stapled anastomosis, the donuts of the EEA-31 hemorrhoid stapler add an extra 16 mm margin in addition to the original specimen (64). LRs are not well reported in the TaTME cohorts. Overall, 17 LRs were reported with a varying follow-up (9.7–29.0 months). The registry has not reported long-term oncological outcome and will probably underreport this fact because of the voluntary non-audited design. The trial data of LaTME reports a 5% LR rate at 3-year follow-up (39).
The lack of long-term outcome underlines the importance of a prospective trial with quality assurance and with auditing of the long-term data. Potentially tumor spill due to inadequate closure of the rectum or due to seeding due to manipulation could be a concern of the TaTME technique.
The cohorts and registry contain unaudited data and publication bias, therefore concern has risen about unreported poor outcomes. Urethral injuries have been mentioned at symposia and training sessions frequently, but fail to be equivalently reported in manuscripts (4,25,69). Other potential injuries such as side wall injury with the risk at major haemorrhage or autonomous nerve injury and a too low stapled anastomosis resulting in poor outcomes are also concerns especially in the learning curve. These potential disastrous complications warrant restraint of wide-spread rigorous implementation of the technique without proper training and auditing (22,70,71).
Although this review contains the most up-to-date overview of the available data substantial limitations are present which precludes any conclusion about the value of TaTME. Only cohort data with selection bias, publication bias and lack of audit. The registry data contains similar bias since data is missing and no audit of the data is present. Comparing RCT with cohort data is only presented due to lack of other comparative data and only serves as an indication. RCT’s often have better results compared to registries since the learning curve is less an issue whereas the TaTME data is biased by a learning curve which has shown to be associated with increased morbidity and worse specimens. The learning curve of laparoscopy has been set at 50–60 patients previous decade, measured by conversion and morbidity (72,73). Koedam et al. analyzed the individual learning curve of a surgeon starting TaTME, and concluded is achieved after 40 cases and 60 more are required to get to the level competent to teach others (Koedam et al. Tech coloproctology 2018, Accepted).
To shorten this learning curve, a training pathway has been designed which covers e-learning, live surgery, hands on cadaver course and on-site proctoring (32,70,71). The international TaTME consensus meeting on the design of a training-pathway concluded due to the technical demanding aspect of TaTME this approach should be reserved for dedicated colorectal surgeons who have extensive experience in both laparoscopic colorectal surgery and TAMIS for local excision (70). The results of the Australian & New Zealand training and implementation program that were included in this review reported the outcomes of 12 surgeons that performed 108 cases TaTME for rectal cancer with a 5.4% anastomotic leak rate, 1.9% CRM+, 0% DRM+ and an intact TME specimen in 107 cases (98.2%) suggesting high quality surgery (49).
Another need is quality assurance to ensure proficiency and safety and avoid patients and results of trials to be hampered by suboptimal performance of not sufficiently trained surgeons (74,75). A well designed trial with these components, in order to capture the real advantage and potential harms of a technique within a training pathway and a patient safety environment, avoids underreporting of poor outcomes and a randomized trial is best suited to rule out bias of excellence centers.
It is well recognized that randomization often discourages patients and surgeons which are in favor of one technique but no other trial design yet has been able to reproduce the level of evidence an adequately powered and executed RCT provides (76).
Conclusions
Continuous cohort reports on TaTME indicate a benefit in conversion rate compared to LaTME and potential increase in sphincter preservation. However, morbidity including anastomotic leakage and by this novel approach introduced specific complications as urethral or pelvic side-wall injury need prospective audit. A significant learning curve is present in the implementation and hampers fair comparison. Long-term oncological outcome does not seem to improve so far but randomized controlled trial with proper quality assurance is best suited to provide data on short-term outcomes as well long-term oncological safety.
The following URL shows supplementary data from the TaTME series: http://ales.amegroups.com/public/system/ales/supp-ales.2018.04.02-2.pdf
Table S1
| Search | Query | Items found |
|---|---|---|
| #1 | Search (((excision*[tiab] OR resection*[tiab] OR TME[tiab] OR TaTME[tiab] OR TAMIS[tiab] OR NOTES[tiab] OR proctectom*[tiab]) AND (transanal*[tiab] OR trans-anal*[tiab])) OR ((excision*[ot] OR resection*[ot] OR TME[ot] OR TaTME[ot] OR TAMIS[ot] OR NOTES[ot] OR proctectom*[ot]) AND (transanal*[ot] OR trans-anal*[ot]))) AND ((((“Neoplasms”[Mesh] OR neoplas*[tw] OR tumor*[tw] OR tumour*[tw] OR cancer*[tw] OR malignan*[tw] OR oncolog*[tw] OR carcinom*[tw] OR adenocarcinom*[tw]) AND (“Rectum”[Mesh] OR rectum[tiab] OR rectal[tiab] OR colorect*[tiab] OR mesorect*[tiab])) AND (“surgery”[Subheading] OR surgery[tiab] OR surgical[tiab] OR operati*[tiab])) OR (“Rectal Neoplasms/surgery”[Mesh:noexp])) | 1,521 |
| #2 | Search #1 AND (“2005/01/01”[Date-Entrez]: “2016/07/01”[Date-Entrez]) | 896 |
| #3 | Search #1 AND (“2005/01/01”[Date-Entrez]: “2018/01/25”[Date-Entrez]) | 1,093 |
| #4 | Search #3 NOT #2 | 197 |
Table S2
| Outcome | ROLARR | COLOR II | ALaCaRT | ACOSOG |
|---|---|---|---|---|
| Inclusion criteria | <15 cm, curative intent, no T4, M0 | <15 cm, cT1-T3, CRM-, no T4, M0 | <15 cm, cT1-T3, CRM-, no T4 | <12 cm, T3N0M0, Tany N1 or 2, M0, and no T4 |
| N | 234 | 699 | 238 | 240 |
| Male sex (%) | 67.9% 159/234 | 64.1% 448/699 | 67.2% 160/238 | 64.5% 156/240 |
| Age mean (SD) | 65.5 (11.93) | 66.8 (10.5) | 65 (56.0–74.0)* | 57.7 (11.5) |
| BMI mean (SD) | NR | 26.1 (4.5) | 27 (24.0–30.0) | 26.4 (4.0) |
| Neoadjuvant therapy | 46.2% 108/234 | 58.9% 412/699 | 50% 119/238 | 98.3% 235/240 |
| Surgical procedure | ||||
| High anterior Resection | 8.3% 19/230 | 10% 72/699 | n.s. | n.s. |
| Low anterior Resection | 71.7% 165/230 | 60% 418/699 | 89% 212/238 | 74.6% 179/240 |
| APR | 19.6% 45/230 | 29% 200/699 | 11% 25/238 | 24.2% 58/240 |
| Missing/other | 0.4% 1/230 | 1% 9/200 | 0.4% 1/238 | 1.2% 3/240 |
| Stoma | ||||
| Temporary | 68.3% 157/230 | 35% 243/690 | 68% 162/238 | 71.3% 171/240 |
| Definitive | 21.3% 49/230 | 34% 236/690 | 13% 30/238 | 26.3% 63/240 |
| No | 10.4% 24/230 | 35% 245/690 | 19% 46/238 | 2.4% 6/240 |
| Quality of the mesorectum (Quirke) | ||||
| Complete | 77.6% 173/230 | 84% 589/690 | 87% 206/238 | 72.9% 175/240 |
| Near/partial | 17.0% 38/230 | 8% 58/690 | 10% 24/238 | 19.2% 46/240 |
| Incomplete | 5.4% 12/230 | 3% 19/690 | 3% 8/238 | 7.9% 19/240 |
| Missing (n) | 7 | 33 | – | – |
| CRM+ | 6.3% 14/224 | 7.3% 43/588 | 6.7% 16/238 | 12.1% 29/240 |
| DRM+ | 0.4% 1/224 | NR | 0.8% 2/238 | 1.7% 4/240 |
| Conversion | 12.2% 28/230 | 16.6% 114/695 | 8.8% 21/238 | 11.2% 27/240 |
| Duration of surgery minutes mean(SD) | 261 (83.24) | 240 (184.0–300.0)* | 210 (163.0–253.0)* | 266.2 (101.9) |
| Intraoperative complications | 14.8% 34/230 | 12% 81/694 | NR | 10.8% 26/240 |
| Bleeding | 4.8% 11/230 | 3% 22/694 | NR | 3.3% 8/240 |
| Length of stay days mean (SD) | 8.2 (6.03) | 8.0 (7.3–8.2)* | 8 (4.44) | 7.3 (5.4) |
| Short-term morbidity | 31.7% 73/230 | 40% 278/697 | NR | 46.3% 111/240 |
| Anastomotic leak | 9.9% 18/181 | 12.6% 58/461 | 3.1% 7/222 | 2.2% 5/240 |
| Mortality (<30 days) | 0.9% 2/230 | 1% 8/699 | 0.6% 1/238 | 0.8% 2/240 |
| Local recurrence (3 yr) | NR | 5% 31/588 | NR | NR |
*, median (inter quartile range). NR, not reported; n.s., not specified; APR, abdominoperineal resection; SD, standard deviation; CRM+, circumferential resection margin involved; DRM+, distal resection margin involved; 3 yr, three years.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.04.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 2010;24:2200-5. [Crossref] [PubMed]
- Sylla P, Rattner DW, Delgado S, et al. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 2010;24:1205-10. [Crossref] [PubMed]
- Simillis C, Hompes R, Penna M, et al. A systematic review of transanal total mesorectal excision: is this the future of rectal cancer surgery? Colorectal Dis 2016;18:19-36. [Crossref] [PubMed]
- Deijen CL, Tsai A, Koedam TW, et al. Clinical outcomes and case volume effect of transanal total mesorectal excision for rectal cancer: a systematic review. Tech Coloproctol 2016;20:811-24. [Crossref] [PubMed]
- Penna M, Hompes R, Arnold S, et al. Transanal Total Mesorectal Excision: International Registry Results of the First 720 Cases. Ann Surg 2017;266:111-7. [Crossref] [PubMed]
- Borstlap WAA, Westerduin E, Aukema TS, et al. Anastomotic Leakage and Chronic Presacral Sinus Formation After Low Anterior Resection: Results From a Large Cross-sectional Study. Ann Surg 2017;266:870-7. [Crossref] [PubMed]
- McDermott FD, Heeney A, Kelly ME, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 2015;102:462-79. [Crossref] [PubMed]
- Jayne D, Pigazzi A, Marshall H, et al. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA 2017;318:1569-80. [Crossref] [PubMed]
- van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Thorpe H, Jayne DG, Guillou PJ, et al. Patient factors influencing conversion from laparoscopically assisted to open surgery for colorectal cancer. Br J Surg 2008;95:199-205. [Crossref] [PubMed]
- Oh SJ, Shin JY. Risk factors of circumferential resection margin involvement in the patients with extraperitoneal rectal cancer. J Korean Surg Soc 2012;82:165-71. [Crossref] [PubMed]
- van der Pas MHGM, Deijen CL, Abis GSA, et al. Conversions in laparoscopic surgery for rectal cancer. Surg Endosc 2017;31:2263-70. [Crossref] [PubMed]
- Yamamoto S, Fukunaga M, Miyajima N, et al. Impact of conversion on surgical outcomes after laparoscopic operation for rectal carcinoma: a retrospective study of 1,073 patients. J Am Coll Surg 2009;208:383-9. [Crossref] [PubMed]
- Agha A, Fürst A, Iesalnieks I, et al. Conversion rate in 300 laparoscopic rectal resections and its influence on morbidity and oncological outcome. Int J Colorectal Dis 2008;23:409-17. [Crossref] [PubMed]
- Allaix ME, Furnée EJ, Mistrangelo M, et al. Conversion of laparoscopic colorectal resection for cancer: What is the impact on short-term outcomes and survival? World J Gastroenterol 2016;22:8304-13. [Crossref] [PubMed]
- Bordeianou L, Maguire LH, Alavi K, et al. Sphincter-sparing surgery in patients with low-lying rectal cancer: techniques, oncologic outcomes, and functional results. J Gastrointest Surg 2014;18:1358-72. [Crossref] [PubMed]
- Borstlap WA, Tanis PJ, Koedam TW, et al. A multi-centred randomised trial of radical surgery versus adjuvant chemoradiotherapy after local excision for early rectal cancer. BMC Cancer 2016;16:513. [Crossref] [PubMed]
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479-82. [Crossref] [PubMed]
- Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007;246:693-701. [Crossref] [PubMed]
- Rickles AS, Dietz DW, Chang GJ, et al. High Rate of Positive Circumferential Resection Margins Following Rectal Cancer Surgery: A Call to Action. Ann Surg 2015;262:891-8. [Crossref] [PubMed]
- Bondeven P, Hagemann-Madsen RH, Laurberg S, et al. Extent and completeness of mesorectal excision evaluated by postoperative magnetic resonance imaging. Br J Surg 2013;100:1357-67. [Crossref] [PubMed]
- Adamina M, Buchs NC, Penna M, et al. St.Gallen consensus on safe implementation of transanal total mesorectal excision. Surg Endosc 2018;32:1091-103. [Crossref] [PubMed]
- Kneist W, Wachter N, Paschold M, et al. Midterm functional results of taTME with neuromapping for low rectal cancer. Tech Coloproctol 2016;20:41-9. [Crossref] [PubMed]
- Arroyave MC, DeLacy FB, Lacy AM. Transanal total mesorectal excision (TaTME) for rectal cancer: Step by step description of the surgical technique for a two-teams approach. Eur J Surg Oncol 2017;43:502-5. [Crossref] [PubMed]
- Penna M, Hompes R, Arnold S, et al. Incidence and Risk Factors for Anastomotic Failure in 1594 Patients Treated by Transanal Total Mesorectal Excision: Results From the International TaTME Registry. Ann Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Dumont F, Goéré D, Honoré C, et al. Transanal endoscopic total mesorectal excision combined with single-port laparoscopy. Dis Colon Rectum 2012;55:996-1001. [Crossref] [PubMed]
- Sylla P, Bordeianou LG, Berger D, et al. A pilot study of natural orifice transanal endoscopic total mesorectal excision with laparoscopic assistance for rectal cancer. Surg Endosc 2013;27:3396-405. [Crossref] [PubMed]
- Zhang H, Zhang YS, Jin XW, et al. Transanal single-port laparoscopic total mesorectal excision in the treatment of rectal cancer. Tech Coloproctol 2013;17:117-23. [Crossref] [PubMed]
- Meng W, Lau K. Synchronous laparoscopic low anterior and transanal endoscopic microsurgery total mesorectal resection. Minim Invasive Ther Allied Technol 2014;23:70-3. [Crossref] [PubMed]
- Zorron R, Phillips HN, Wynn G, et al. "Down-to-Up" transanal NOTES Total mesorectal excision for rectal cancer: Preliminary series of 9 patients. J Minim Access Surg 2014;10:144-50. [Crossref] [PubMed]
- Elmore U, Fumagalli Romario U, Vignali A, et al. Laparoscopic anterior resection with transanal total mesorectal excision for rectal cancer: preliminary experience and impact on postoperative bowel function. J Laparoendosc Adv Surg Tech A 2015;25:364-9. [Crossref] [PubMed]
- McLemore EC, Harnsberger CR, Broderick RC, et al. Transanal total mesorectal excision (taTME) for rectal cancer: a training pathway. Surg Endosc 2016;30:4130-5. [Crossref] [PubMed]
- Foo DC, Choi HK, Wei R, et al. Transanal Total Mesorectal Excision With Single-Incision Laparoscopy for Rectal Cancer. JSLS 2016;20: [Crossref] [PubMed]
- Marks JH, Lopez-Acevedo N, Krishnan B, et al. True NOTES TME resection with splenic flexure release, high ligation of IMA, and side-to-end hand-sewn coloanal anastomosis. Surg Endosc 2016;30:4626-31. [Crossref] [PubMed]
- Lelong B, Meillat H, Zemmour C, et al. Short- and Mid-Term Outcomes after Endoscopic Transanal or Laparoscopic Transabdominal Total Mesorectal Excision for Low Rectal Cancer: A Single Institutional Case-Control Study. J Am Coll Surg 2017;224:917-25. [Crossref] [PubMed]
- Yao HW, Wu GC, Yang YC, et al. Laparoscopic-assisted Transanal Total Mesorectal Excision for Middle-Low Rectal Carcinoma: A Clinical Study of 19 Cases. Anticancer Res 2017;37:4599-604. [PubMed]
- Lacy AM, Tasende MM, Delgado S, et al. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J Am Coll Surg 2015;221:415-23. [Crossref] [PubMed]
- de Lacy FB, van Laarhoven JJEM, Pena R, et al. Transanal total mesorectal excision: pathological results of 186 patients with mid and low rectal cancer. Surg Endosc 2017; [Epub ahead of print]. [PubMed]
- Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;372:1324-32. [Crossref] [PubMed]
- Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015;314:1356-63. [Crossref] [PubMed]
- Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015;314:1346-55. [Crossref] [PubMed]
- Hüscher CG, Tierno SM, Romeo V, et al. Technologies, technical steps, and early postoperative results of transanal TME. Minim Invasive Ther Allied Technol 2016;25:247-56. [Crossref] [PubMed]
- Perdawood SK, Thinggaard BS, Bjoern MX. Effect of transanal total mesorectal excision for rectal cancer: comparison of short-term outcomes with laparoscopic and open surgeries. Surg Endosc 2017; [Epub ahead of print]. [PubMed]
- Maykel JA, Phatak UR, Suwanabol PA, et al. Initiation of a Transanal Total Mesorectal Excision Program at an Academic Training Program: Evaluating Patient Safety and Quality Outcomes. Dis Colon Rectum 2017;60:1267-72. [Crossref] [PubMed]
- Caycedo-Marulanda A, Jiang HY, Kohtakangas EL. Outcomes of a Single Surgeon-Based Transanal-Total Mesorectal Excision (TATME) for Rectal Cancer. J Gastrointest Cancer 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Gilshtein H, Neymark M, Manassa E, et al. Transanal Endoscopic Microsurgery Implementation in Ultra-Low Anterior Resection. J Laparoendosc Adv Surg Tech A 2018;28:186-8. [Crossref] [PubMed]
- Chang TC, Kiu KT. Transanal Total Mesorectal Excision in Lower Rectal Cancer: Comparison of Short-Term Outcomes with Conventional Laparoscopic Total Mesorectal Excision. J Laparoendosc Adv Surg Tech A 2017; [Epub ahead of print]. [PubMed]
- Perez D, Melling N, Biebl M, et al. Robotic low anterior resection versus transanal total mesorectal excision in rectal cancer: A comparison of 115 cases. Eur J Surg Oncol 2018;44:237-42. [Crossref] [PubMed]
- Abbott SC, Stevenson ARL, Bell SW, et al. An assessment of an Australasian pathway for the introduction of transanal total mesorectal excision (taTME). Colorectal Dis 2018;20:O1-O6. [Crossref] [PubMed]
- Buchs NC, Wynn G, Austin R, et al. A two-centre experience of transanal total mesorectal excision. Colorectal Dis 2016;18:1154-61. [Crossref] [PubMed]
- Burke JP, Martin-Perez B, Khan A, et al. Transanal total mesorectal excision for rectal cancer: early outcomes in 50 consecutive patients. Colorectal Dis 2016;18:570-7. [Crossref] [PubMed]
- Chen CC, Lai YL, Jiang JK, et al. Transanal Total Mesorectal Excision Versus Laparoscopic Surgery for Rectal Cancer Receiving Neoadjuvant Chemoradiation: A Matched Case-Control Study. Ann Surg Oncol 2016;23:1169-76. [Crossref] [PubMed]
- Chouillard E, Chahine E, Khoury G, et al. NOTES total mesorectal excision (TME) for patients with rectal neoplasia: a preliminary experience. Surg Endosc 2014;28:3150-7. [Crossref] [PubMed]
- Muratore A, Mellano A, Marsanic P, et al. Transanal total mesorectal excision (taTME) for cancer located in the lower rectum: short- and mid-term results. Eur J Surg Oncol 2015;41:478-83. [Crossref] [PubMed]
- Perdawood SK, Al Khefagie GA. Transanal vs laparoscopic total mesorectal excision for rectal cancer: initial experience from Denmark. Colorectal Dis 2016;18:51-8. [Crossref] [PubMed]
- Procházka V, Kala Z, Škrovina M, et al. Transanal total mesorectal excision for low rectal cancer - first results. Rozhl Chir 2015;94:64-8. [PubMed]
- Rasulov AO, Mamedli ZZ, Gordeyev SS, et al. Short-term outcomes after transanal and laparoscopic total mesorectal excision for rectal cancer. Tech Coloproctol 2016;20:227-34. [Crossref] [PubMed]
- Rink AD, Kauff DW, Paschold M, et al. Hybrid TAMIS total mesorectal excision. A new perspective in treatment of distal rectal cancer - Technique and results. Chirurg 2016;87:225-32. [Crossref] [PubMed]
- Rouanet P, Mourregot A, Azar CC, et al. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum 2013;56:408-15. [Crossref] [PubMed]
- Serra-Aracil X, Mora-López L, Casalots A, et al. Hybrid NOTES: TEO for transanal total mesorectal excision: intracorporeal resection and anastomosis. Surg Endosc 2016;30:346-54. [Crossref] [PubMed]
- Tuech JJ, Karoui M, Lelong B, et al. A step toward NOTES total mesorectal excision for rectal cancer: endoscopic transanal proctectomy. Ann Surg 2015;261:228-33. [Crossref] [PubMed]
- Veltcamp Helbach M, Deijen CL, Velthuis S, et al. Transanal total mesorectal excision for rectal carcinoma: short-term outcomes and experience after 80 cases. Surg Endosc 2016;30:464-70. [Crossref] [PubMed]
- Deijen CL, Velthuis S, Tsai A, et al. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc 2016;30:3210-5. [Crossref] [PubMed]
- Penna M, Knol JJ, Tuynman JB, et al. Four anastomotic techniques following transanal total mesorectal excision (TaTME). Tech Coloproctol 2016;20:185-91. [Crossref] [PubMed]
- Velthuis S, Veltcamp Helbach M, Tuynman JB, et al. Intra-abdominal bacterial contamination in TAMIS total mesorectal excision for rectal carcinoma: a prospective study. Surg Endosc 2015;29:3319-23. [Crossref] [PubMed]
- Penna M, Cunningham C, Hompes R. Transanal Total Mesorectal Excision: Why, When, and How. Clin Colon Rectal Surg 2017;30:339-45. [Crossref] [PubMed]
- Nagtegaal ID, Marijnen CA, Kranenbarg EK, et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol 2002;26:350-7. [Crossref] [PubMed]
- Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009;373:821-8. [Crossref] [PubMed]
- Atallah SB, DuBose AC, Burke JP, et al. Uptake of Transanal Total Mesorectal Excision in North America: Initial Assessment of a Structured Training Program and the Experience of Delegate Surgeons. Dis Colon Rectum 2017;60:1023-31. [Crossref] [PubMed]
- Francis N, Penna M, Mackenzie H, et al. Consensus on structured training curriculum for transanal total mesorectal excision (TaTME). Surg Endosc 2017;31:2711-9. [Crossref] [PubMed]
- Penna M, Hompes R, Mackenzie H, et al. First international training and assessment consensus workshop on transanal total mesorectal excision (taTME). Tech Coloproctol 2016;20:343-52. [Crossref] [PubMed]
- Tekkis PP, Senagore AJ, Delaney CP, et al. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 2005;242:83-91. [Crossref] [PubMed]
- Bege T, Lelong B, Esterni B, et al. The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution's experience. Ann Surg 2010;251:249-53. [Crossref] [PubMed]
- Foster JD, Mackenzie H, Nelson H, et al. Methods of quality assurance in multicenter trials in laparoscopic colorectal surgery: a systematic review. Ann Surg 2014;260:220-9. [Crossref] [PubMed]
- Miskovic D, Ni M, Wyles SM, et al. Is competency assessment at the specialist level achievable? A study for the national training programme in laparoscopic colorectal surgery in England. Ann Surg 2013;257:476-82. [Crossref] [PubMed]
- Torgerson DJ, Sibbald B. Understanding controlled trials. What is a patient preference trial? BMJ 1998;316:360. [Crossref] [PubMed]
Cite this article as: van Oostendorp SE, Koedam TWA, Sietses C, Bonjer HJ, Tuynman JB. Transanal total mesorectal excision compared to laparoscopic TME for mid and low rectal cancer—current evidence. Ann Laparosc Endosc Surg 2018;3:41.


