The development of robotic hepatopancreatobiliary surgery in Singapore: a multi-institutional experience
Introduction
Since the introduction of laparoscopy the early 1900s, minimally invasive surgery (MIS) has revolutionized the practice of general surgery over the past century (1). Prior to Mouret’s first laparoscopic cholecystectomy in 1987, a generous Kocher’s or midline incision was common practice in the management of gallstone diseases (2). At present, MIS hepatopancreatobiliary (HPB) surgery has evolved to become standard of care amongst specialist units worldwide (3). International expert committees have concluded laparoscopic liver resection to be safe and oncologically non-inferior to open hepatectomy while providing benefits of a MIS approach (4,5). While no randomized control trials (RCTs) exist, laparoscopic pancreatic surgery have also gained widespread acceptance in high volume tertiary centers where morbidity and survival outcomes seem to parallel its open counterparts (6,7).
Despite the success of conventional laparoscopy, Nguyen et al. reported that only a minority of major hepatectomy are attempted via a laparoscopic approach with 84% of all laparoscopic liver resections limited to wedge or lateral sectionectomies (8). In fact, though feasible and safe, laparoscopic distal pancreatectomies have been associated with a relatively high conversion rate of 38% even in high volume specialist units (7). While disappointing, these results were not surprising given the steep learning curve and inherent limitations of conventional laparoscopy—restricted range of motion, physiological tremors, awkward surgeon ergonomics, poor visualization and depth perception (9).
Robotic operating systems, crowned as the new frontier of MIS, seek to negate the disadvantages of its laparoscopic predecessor. The Da Vinci platform have since dominated the field of robotic surgery by providing intuitive and articulated robotic arms which allowed the performance of complex and technically demanding intra-abdominal surgeries (10).
In Singapore, the first robotic assisted HPB surgery was performed in 2013 (11). Favorable early results prompted the rolling out of robotic programs nationwide. By 2015, all 4 tertiary institutions have acquired the da Vinci operating platform and were actively engaging in robotic urological, colorectal, gynecological and HPB surgeries (11-14). With the widespread acceptance of the robotic approach, we aim to evaluate the trends in its early development and the safety and feasibility in major HPB surgeries.
Methods
Study design
A questionnaire was designed and collected from all accredited participating HPB specialist surgeons in Singapore. Surgery related data was collected based on each surgeon’s experience from January 2013 to February 2018. These included: type of HPB surgery performed, intra-operative blood loss, operation duration, rates of conversion as well as post-operative morbidity and mortality.
Robotic surgery in Singapore
Robotic HPB surgery was performed in 2 tertiary centers nationwide.
Singapore General Hospital
Robotic HPB procedures were performed using the Da Vinci-Si Surgical System (Intuitive Surgical, Sunnyvalle, CA, USA). All surgeries were performed by three principal surgeons in the HPB unit.
Changi General Hospital
Robotic HPB procedures were performed using the Da Vinci-Xi Surgical System (Intuitive Surgical). All surgeries were performed by two principal HPB surgeons.
As our study represents the most updated results of all robotic HPB cases performed in Singapore, it may contain overlapping patient data that have been previously published (15-17).
Results
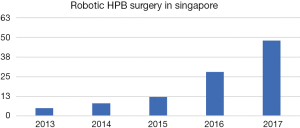
A total of 112 robotic HPB procedures were performed in 2 specialist units over a 5-year and 2-month duration. Since its introduction, the number of patients undergoing robotic surgery has increased exponentially over the years (Figure 1).
Robotic liver resection
Forty-seven patients underwent robotic hepatectomies (RH) during the study duration. Anatomical liver resection was more commonly performed than non-anatomical wedge resection (63.8% vs. 36.2%). Majority of the cases (n=38, 80.9%) were minor RH (<3 liver segments); 9 (19.1%) patients underwent major RH (Table 1). Twenty-one (44.7%) had tumors located at the posterosuperior segments. Eight (17%) RH patients underwent concomitant abdominal surgery, which included: 3 anterior resections for colorectal cancer with synchronous liver metastases, 3 hilar lymphadenectomies for gallbladder cancer, 1 right portal vein ligation prior to the second stage hepatectomy for bilobar colorectal liver metastases and 1 choledochectomy with hepaticojejunostomy (HJ) for cholangiocarcinoma. Thirty-six cases were found to have malignant tumor on final histology, of which 21 (45%) patients had hepatocellular carcinoma (HCC).
Table 1
| Type of resection | No. of patients (N=47) |
|---|---|
| Major hepatectomy (>3 segments) | 9 (19.1%) |
| Minor hepatectomy | 21 (44.7%) |
| Left lateral sectionectomy | 6 |
| Right posterior sectionectomy | 8 |
| Right anterior sectionectomy | 1 |
| Left medial sectionectomy | 1 |
| Anatomical sectionectomy (others) | 5 |
| Wedge resections | 17 (36.2%) |
Operative morbidity
The median operative time was 366 (range, 75–885) minutes and the median blood loss was 300 (range, 20–4,500) mL. There were 3 conversions during RH: 1 (2.1%) open conversion was due to bleeding and the other 2 (4.2%) were converted to hand-assist due to poor port placement during our initial experience. The median length of hospital stay was 5 (range, 2–33) days. There were 3 (6.4%) patients with major morbidity (> Clavein-Dindo grade 2): 2 patients had infected intra-abdominal collections requiring percutaneous drainage; the third patient suffered an anastomotic leak after robotic anterior resection and left lateral sectionectomy was performed for synchronous colorectal liver metastases for which a laparotomy was performed.
Robotic pancreas surgery
A total of 46 robotic pancreatic resections were performed (Table 2). There were 4 (8.7%) conversions to open: 3 were due to locally advanced tumors and one was due to bleeding. The median operative time was 525 (range, 200–930) minutes.
Table 2
| Type of pancreatectomy | No. of patients (N=46) |
|---|---|
| Pancreaticoduodenectomy (PD) | 18 (39.1%) |
| Distal pancreatectomy (DP) | 25 (54.3%) |
| Spleen preserving | 12 (1 Warshaw’s technique) |
| Splenectomy | 13 (7 subtotal DP) |
| Enucleation | 2 (4.3%) |
| Peustow procedure | 1 (2.2%) |
Pancreaticoduodenectomy (PD)
Of the 18 PDs, 5 were hybrid procedures where resection was performed robotically and reconstruction performed using a small upper midline incision. Three PDs had extended vascular resection and reconstruction to achieve R0 margins. One patient had concomitant robotic assisted right partial nephrectomy for a renal cell carcinoma—this was performed with the Da Vinci-Xi Surgical System.
There was no grade B postoperative pancreatic fistula (POPF) in the PD group. Five patients (27.8%) had major morbidity, which included 2 patients (11.1%) with grade C POPF requiring laparotomy. The 2 cases required completion pancreatectomy and ligation of pancreatic duct respectively. Two patients (11.1%) had delayed gastric emptying requiring nasojejunostomy tube (NJT) insertion for feeding, and 1 (5.6%) with bleeding at the gastrojejunostomy requiring gastroscopy evaluation and clipping of the bleeding site. There was no 30- or 90-day mortality, however, there was 1 (5.6%) in-hospital mortality from cardiorespiratory failure with bilateral pneumonia which occurred after 90 days post operatively from a patient with grade C POPF.
Distal pancreatectomy (DP)
In the DP group (n=25), 12 (48%) patients had spleen-preserving DP of which only one case was performed by Warshaw technique. The remaining 13 (52%) patients had DP with splenectomy, 7 (28%) of which had subtotal DP for tumors located at the body or neck of the pancreas. Four (16%) patients suffered major morbidity, all due to grade B POPF. There was no grade C POPF or 90-day mortality.
Others
Robotic pancreatic enucleation was performed for 2 patients with pancreatic neuroendocrine tumor and 1 robotic Peustow procedure for chronic pancreatitis. One patient who underwent enucleation had a grade B POPF.
Robotic biliary surgery
Nineteen (17%) of the robotic cases were biliary in nature. The use of the robot for biliary surgery was reserved for complex cases. These included a triple bypass for locally advanced unresectable pancreatic cancer, cholecystectomy with common bile duct exploration, cholecystectomy for Mirizzi’s syndrome, resection of choledochal cyst with HJ anastomosis and radical cholecystectomy with segment 4b and 5 liver resection for gall bladder carcinoma. Four patients who had biliary procedure had concomitant liver resections which were also included under the robotic liver resection. Robotic cholecystectomy was not performed for biliary colic due to gallstones.
Discussion
The initial development of robotic technology in surgery was driven by its potential military applications. The proposed master-slave platform enabled surgeons to remotely reproduce hand movements via laparoscopic instruments intra-corporeally (10). Subsequent modifications led to the introduction of the Da Vinci platform which boost improved dexterity, precision, visual magnification, three-dimensional views as well as decreased tremors compared with conventional laparoscopic surgery (18,19). In 1997, the world’s first robotic surgery—‘tele-surgical’ cholecystectomy was performed using the da Vinci system (20). To date, the application of robotics in surgery have been ubiquitous and include maxillofacial, cardiac, thoracic, urological, gynecologic, and in general surgery (21).
Unlike its colorectal and urological counterparts, the progression and adoption of minimally invasive techniques in major HPB surgery has been relatively slow (8). Despite international consensus and strong evidence proving the safety and oncological equivalence of laparoscopic to open hepatectomies (22-24), the use of laparoscopy in complex liver surgeries remains limited in view of inherent limitations of laparoscopic surgery (9). Since the first reported robotic-assisted liver resection in 2006 (25), the application of the robot in both minor and major hepatectomies have seen a tremendous increase with 537 reported cases of RH performed between 2006 to 2014 (26). In Singapore, we found a similar increasing trend in the number of robotic liver surgery performed between 2013 and 2017 (Figure 1). Supporters of the robotic system describe the wristed robotic arm with seven degrees of freedom similar to the human hand, providing a greater degree of dexterity and accuracy. Furthermore, magnified three-dimensional surgical views from the surgeon controlled robotic camera allowed for depth perception such that delicate tissue dissection and precise intra-corporeal suturing may be performed (27). This is especially important in major hepatic surgery where adequate control of bleeding vessels in tight spaces is paramount to prevent conversion to an open surgical procedure (10). Tsung et al. further reported higher rates of successful purely minimally invasive approach with the robotic technique when compared with conventional laparoscopy for major hepatectomies (28).
In a matched comparison study, the Taiwanese group found the robotic arm reproducing results of open surgery in its performance in complex major resections even in cirrhotic livers. No difference in intraoperative blood loss, postoperative complications, resection margins or survival outcomes was found (28). In fact, patients who underwent robotic surgery experienced less postoperative pain, earlier ambulation and a shortened hospital stay (29). Based on our national survey, a 6% conversion rate was seen in RH and median length of hospital stay was 5 days. These results compare well with our international counterparts. Furthermore, 8 patients received concomitant abdominal surgery in addition to liver resection via the robotic platform, this would have been challenging with conventional laparoscopy. Therefore, we believe that the robot may indeed provide superior technical advantages over its laparoscopic counterpart that may allow for complex surgical procedures to be performed more efficiently.
Pancreatic surgery has been touted as one of the most complex intra-abdominal procedure associated with significant rates of morbidity and mortality (30-32). Pancreaticoduodenectomy, first performed successfully for periampullary cancer by William Steward Halsted in 1898, poses the greatest challenge when attempted via a minimally invasive approach (32). While laparoscopic PD has been proven safe and feasible (33), it is the robotic approach that has shown lower conversion rates with majority completed via a purely MIS fashion (34,35). Advantages were replicated in robotic DP where higher rate of splenic preservation, reduced rate of conversion and length of hospital stay were reported (36). While our national experience with robotic pancreatic surgery is limited, we have managed to complete 80.4% of our cases via a purely MIS approach. Morbidity was often a result of POPF though the rate appeared to be similar with our open experience.
Robotic surgery, however, is not without its limitations. Prohibitive cost is a significant barrier to its implementation in centers worldwide. When coupled with longer operating room time, the costs of purchase and maintenance, economic burden of the robotic approach further increases. Without strong level 1 evidence proving significant advantages of the robot over laparoscopy, universal adoption remains a challenge. However, as surgeons nationwide gain further experience with the intuitive robotic platform, its stability and ease in usage will eventually translate to improved patient safety and lowered morbidity rates. As such, the long-term benefits of this promising technology should not be underestimated.
Conclusions
There has been a gradual but steady increase in the number of robotic HPB surgeries performed in Singapore. Our national early experience demonstrated that the robotic platform is a safe and feasible option that may be utilized with minimal morbidity especially for complex HPB surgeries.
Acknowledgments
This research was supported by the SingHealth Group.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery for the series “Advanced Laparoscopic HPB Surgery in South East Asia”. The article has undergone external peer review.
Conflicts of Interest: The series “Advanced Laparoscopic HPB Surgery in South East Asia” was commissioned by the editorial office without any funding or sponsorship. AKHC, SYL and CYC served as the unpaid Guest Editor of the series. BKG has received travel support and proctor fees from Transmedic, the local distributor of the Da Vinci robotic system in Singapore. SYL, CYC, JYT have received travel support from Transmedic. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The individual institutions have IRB approval for data collection of these patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vecchio R, MacFayden BV, Palazzo F. History of laparoscopic surgery. Panminerva Med 2000;42:87-90. [PubMed]
- Mouret P. How I developed laparoscopic cholecystectomy. Ann Acad Med Singapore 1996;25:744-7. [PubMed]
- Barkun JS, Barkun AN, Sampalis JS, et al. Randomised controlled trials of laparoscopic versus mini cholecystectomy. Lancet 1992;340:1116-9. [Crossref] [PubMed]
- Cheung TT, Han HS, She WH, et al. The Asia Pacific Consensus Statement on Laparoscopic Liver Resection for Hepatocellular Carcinoma: A Report from the 7th Asia-Pacific Primary Liver Cancer Expert Meeting Held in Hong Kong. Liver Cancer 2018;7:28-39. [Crossref] [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [PubMed]
- Pędziwiatr M, Małczak P, Pisarska M, et al. Minimally invasive versus open pancreatoduodenectomy-systematic review and meta-analysis. Langenbecks Arch Surg 2017;402:841-51. [Crossref] [PubMed]
- Lee SY, Allen PJ, Sadot E, et al. Distal pancreatectomy: a single institution’s experience in open, laparoscopic, and robotic approaches. J Am Coll Surg 2015;220:18-27. [Crossref] [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [Crossref] [PubMed]
- Bonapasta SA, Bartolini I, Checcacci P, et al. Indications for liver surgery: laparoscopic or robotic approach. Updates Surg 2015;67:117-22. [Crossref] [PubMed]
- Marino MV, Shabat G, Gulotta G, et al. From Illusion to Reality: A Brief History of Robotic Surgery. Surg Innov 2018;25:291-6. [Crossref] [PubMed]
- Goh BK, Wong JS, Chan CY, et al. First experience with robotic spleen-saving, vessel-preserving distal pancreatectomy in Singapore: report of three consecutive cases. Singapore Med J 2016;57:464-9. [Crossref] [PubMed]
- Bang SL, Png KS, Yeow YY, et al. Developing technical expertise in robot-assisted laparoscopic prostatectomy in a moderate-volume center through a proctor-based team approach. J Robot Surg 2014;8:245-50. [Crossref] [PubMed]
- Ng JS, Fong YF, Tong PS, et al. Gynaecologic robot-assisted cancer and endoscopic surgery (GRACES) in a tertiary referral centre. Ann Acad Med Singapore 2011;40:208-12. [PubMed]
- Eu EW, Ngu JC, Chiow AKH. How to do a combined robotic anterior resection and liver resection: da Vinci Xi. ANZ J Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Goh BKP, Low TY, Lee SY, et al. Initial experience with robotic pancreatic surgery in Singapore: single institution experience with 30 consecutive cases. ANZ J Surg 2018; [Epub ahead of print]. [PubMed]
- Goh BKP, Lee LS, Lee SY, et al. Initial experience with robotic hepatectomy in Singapore: analysis of 48 resections in 43 consecutive patients. ANZ J Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Goh BK, Lee SY, Chan CY, et al. Early experience with robot-assisted laparoscopic hepatobiliary and pancreatic surgery in Singapore: single-institution experience with 20 consecutive patients. Singapore Med J 2018;59:133-8. [Crossref] [PubMed]
- Ballantyne GH. The pitfalls of laparoscopic surgery: challenges for robotics and telerobotic surgery. Surg Laparosc Endosc Percutan Tech 2002;12:1-5. [Crossref] [PubMed]
- Wilson EB. The evolution of robotic general surgery. Scand J Surg 2009;98:125-9. [Crossref] [PubMed]
- Himpens J, Leman G, Cadiere GB. Telesurgical laparoscopic cholecystectomy. Surg Endosc 1998;12:1091. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Jin B, Chen MT, Fei YT, et al. Safety and efficacy for laparoscopic versus open hepatectomy: A meta-analysis. Surg Oncol 2018;27:A26-34. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg 2018;267:199-207. [Crossref] [PubMed]
- Ryska M, Fronek J, Rudis J, et al. Manual and robotic laparoscopic liver resection. Two case-reviews Rozhl Chir 2006;85:511-6. [in Czech]. [PubMed]
- Qiu J, Chen S, Chengyou D. A systematic review of robotic-assisted liver resection and meta-analysis of robotic versus laparoscopic hepatectomy for hepatic neoplasms. Surg Endosc 2016;30:862-75. [Crossref] [PubMed]
- Hanna T, Imber C. Robotics in HPB surgery. Ann R Coll Surg Engl 2018;100:31-7. [Crossref] [PubMed]
- Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549-55. [Crossref] [PubMed]
- Chen PD, Wu CY, Hu RH, et al. Robotic Versus Open Hepatectomy for Hepatocellular Carcinoma: A Matched Comparison. Ann Surg Oncol 2017;24:1021-8. [Crossref] [PubMed]
- Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006;244:10-5. [Crossref] [PubMed]
- Valente R, Lykoudis P, Tamburrino D, et al. Major postoperative complications after pancreatic resection for P-NETS are not associated to earlier recurrence. Eur J Surg Oncol 2017;43:2119-28. [Crossref] [PubMed]
- Halsted WS. Contributions to the surgery of the bile passages, especially of the common bile duct. Boston Med Surg J 1899;141:645-54. [Crossref]
- Coppola A, Stauffer JA, Asbun HJ. Laparoscopic pancreatoduodenectomy: current status and future directions. Updates Surg 2016;68:217-24. [Crossref] [PubMed]
- Zureikat AH, Borrebach J, Pitt HA, et al. Minimally invasive hepatopancreatobiliary surgery in North America: an ACS-NSQIP analysis of predictors of conversion for laparoscopic and robotic pancreatectomy and hepatectomy. HPB 2017;19:595-602. [Crossref] [PubMed]
- Cirocchi R, Partelli S, Trastulli S, et al. A systematic review on robotic pancreaticoduodenectomy. Surg Oncol 2013;22:238-46. [Crossref] [PubMed]
- Guerrini GP, Lauretta A, Belluco C, et al. Robotic versus laparoscopic distal pancreatectomy: an up-to-date meta-analysis. BMC Surg 2017;17:105. [Crossref] [PubMed]
Cite this article as: Lee LS, Wong JSM, Chiow AKH, Lee SY, Chan CY, San Tan S, Teo JY, Goh BKP. The development of robotic hepatopancreatobiliary surgery in Singapore: a multi-institutional experience. Ann Laparosc Endosc Surg 2018;3:59.