Laparoscopic liver resection in Malaysia—a single surgeon’s learning curve
Introduction
Although the first laparoscopic liver resection (LLR) was reported by Gagner et al. [1992] (1), LLR was not embraced as rapidly as the other laparoscopic surgery due to some technical difficulties such as the fear of uncontrollable hemorrhage and air embolism. This has led to the surgical community being restrained and hesitant towards the adoption of this approach especially in major hepatectomy (2,3). Nearly 10 years after the introduction of LLR, the first clearly defined series of LLRs were reported by Cherqui et al. [2000] and Descottes et al. [2000] (4,5). Its use has increased rapidly along with the advancement of biomedical technology (e.g., intra-operative use of ultrasound, advanced hemostatic techniques) and improvement in surgical techniques (6). The evolution of LLR brought upon the “Louisville Statement” in 2008, in which LLR was recognized as an effective and safe approach with acceptable morbidity and mortality for minor and major liver resections by surgeons experienced in hepatobiliary and laparoscopic surgery (7). A new scoring system was proposed by Ban et al. (8) to evaluate the difficulty of different laparoscopic hepatectomy procedures, included a difficulty score ranging from 1–10 based on five components: location of tumour, extent of resection, size of tumour, proximity to major vessels, and liver function (Child-Pugh). Based on this scoring system, surgeons can overcome the learning curve and perform LLR with stepwise approach based on the difficulty of the procedure and the experiences of each individual surgeon.
As of 2016, there are more than 9,500 LLR cases reported worldwide (9). However, there is little published data on the outcomes of LLR in the South East Asian (SEA) region. This retrospective study aimed to evaluate and report the outcomes of LLR from a single-surgeon’s experience in Malaysia.
Methods
Patient & data collection
The medical records of patients who underwent LLR by a single surgeon were retrospectively reviewed between October 2009 and December 2017. Ethical approval was obtained from Sunway Medical Centre Independent Research Ethics Committee.
Data collection included demographics (age and gender), pathological diagnoses, types of resections, perioperative parameters (operation time, blood loss, and blood transfusion), conversion rate, postoperative length of stay (LOS), postoperative complications and interventional procedures, and mortality. “Major resection” was defined as the resection of at least three liver segments. “Minor resection” was defined as the resection of one or two liver segments (10). The anterolateral segments (“Favourable location”) are denoted as segments 2, 3, 4b, 5, 6 while the posterosuperior segments (“Unfavourable location”) are defined as segments 1, 4a, 7, 8 (11,12). Operative mortality referred to the occurrence of death postoperatively within 30 days.
The severity of the complications was scored using the Clavien-Dindo classification (13), where Grades I and II indicated no or minor complications, whereas Grades IIIa to IVb indicated severe complications to multiorgan dysfunction and Grade V indicated death. Cases of liver resection were categorised according to the Couinaud’s classification (14). The difficulty of LLR was graded using the scoring system proposed by Ban et al. (8). Cumulative sum control chart (CUSUM) analysis was applied to the difficulty scoring to assess the learning curve’s progression and evolution over time (15,16).
Statistical analysis
Data collection was performed with the use of the Microsoft Excel program. Quantitative data were described in the form of mean, median and range. Qualitative data were described in the form of frequency or percentage.
Results
There were 23 females and 21 males in this study, with a median age of 60.5 years (range, 29–79 years). The number of patients who underwent LLR from October 2009 to November 2017 were 1 in 2009, 14 in 2010, 7 in 2011, 1 in 2012, 3 in 2013, 2 in 2014, 6 in 2015, 6 in 2016, and 4 in 2017, respectively. Patients’ demographic data and pathological diagnoses are summarized in Table 1.
Table 1
| Variable | Patients (n=44) |
|---|---|
| Sex (M/F) | 21/23 |
| Age (year) | 60.5 [29–79] |
| Pathologic diagnoses | |
| Malignant | |
| Hepatocellular carcinoma | 16 |
| Colorectal liver metastasis | 8 |
| Cholangiocarcinoma | 2 |
| Gallbladder adenocarcinoma | 1 |
| Endometrial liver metastasis | 1 |
| Metastatic renal cell carcinoma | 1 |
| Non-malignant | |
| Hemangioma | 5 |
| Focal nodular hyperplasia | 3 |
| Complex liver cysts | 2 |
| Nodular hepatic steatosis | 1 |
| Cholangio-hepatolithiasis | 1 |
| Suspected gallbladder carcinoma | 1 |
| Pyogenic liver abscess | 1 |
| Intrahepatic splenic tissue | 1 |
| Tumour size (mm) | 40 [6–180] |
| Surgical resections (mm) | 12 [1–45] |
| Positive resection margin (R1) | 1 |
Patients’ operative details are summarized in Table 2. The rate of conversion to open surgery was 13.6%. The reasons for conversion were as follows: two patients underwent conversion to open surgery due to profuse bleeding from the portal vein branch. One patient was found to have hepatocellular carcinoma (HCC) directly invaded the diaphragm, which required concurrent diaphragmatic resection and repair. Two patients experienced dense adhesion within the abdomen due to previous abdominal surgery and adhesiolysis was not possible. One patient had a hemangioma lesion that was not well localised at segment 8. The median operative time was 138 min (range, 45–344 min). The median estimated blood loss was 200 mL (range, 50–2,000 mL) and 2 (4.5%) patients required intraoperative transfusions. Between these 2 patients, the median number of packed red blood cell units were 1.5 units (range, 1–2 units).
Table 2
| Variable | Patients (n=44) |
|---|---|
| Major/minor resection | 10/34 (22.7%/77.3%) |
| Type of resection | |
| Wedge resection | 18 |
| Left lateral sectionectomy | 14 |
| Left hepatectomy | 6 |
| Left lateral sectionectomy + wedge resection | 4 |
| Bi-segmentectomy | 1 |
| Caudate lobectomy | 1 |
| Techniques of resection | |
| Total laparoscopic surgery | 38 |
| Hand-assisted laparoscopic surgery | 3 |
| SILS | 2 |
| DVSS surgery | 1 |
| Segments | |
| Favourable location (anterolateral) | 35 (79.5%) |
| Unfavourable location (posterosuperior) | 3 (6.8%) |
| Both favourable and unfavourable location | 6 (13.6%) |
| Open conversion (yes/no) | 6/38 (13.6%/86.4%) |
| Reason for conversion | |
| Bleeding | 2 |
| Bleeding + diaphragmatic tumour invasion | 1 |
| Dense adhesion | 2 |
| Poor localization of tumour | 1 |
| Operative time (min) | 138 [45–344] |
| Blood loss (mL) | 200 [50–2,000] |
| Perioperative blood transfusion | 2/44 (4.5%) |
| No. of packed red blood cell units | 1.5 [1–2] |
| Postoperative morbidity | 1 (2.3%) |
| Clavien-Dindo (Grade II) | 1 |
| Postoperative mortality | 0 |
| Surgical complication (urinary retention) | 1 |
| Intervention (urinary catheterization) | 1 |
| Length of stay (days) | 5 [2–12] |
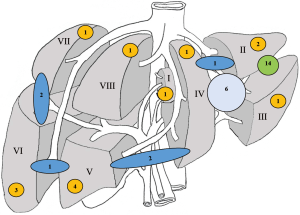
Figure 1 summarized the locations of lesions resected: 77.3% of favourable locations versus 9.1% of unfavourable locations. Four cases were not stated in Figure 1 and they were located at both favourable and unfavourable locations. Two patients underwent laparoscopic left lateral sectionectomy and segment 8 wedge resection while another two patients underwent laparoscopic left lateral sectionectomy and segment 7 wedge resection. Multiple lesions were observed in 20.5% of the cases, and the maximum number of resected lesions was three. Positive resection margin (R1) was observed in 1 (2.3%) of the specimens involving curative-intent surgery for malignant tumours. The median resection margin was 13.5 mm (range, 1–50 mm).
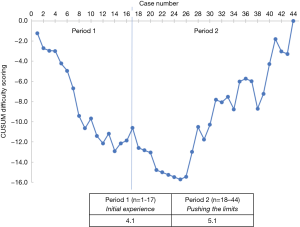
According to the CUSUM analysis of the difficulty scoring, two periods were identified: period 1, n=17 cases; and period 2, from case 18 to case 44, n=27 cases. The average of the overall difficulty scoring was 4.1 for period 1 and 5.1 for period 2 (Figure 2).
During period 1, minor resections were predominantly performed. The median estimated blood loss was 200 mL (range, 11–1,500 mL). The conversion rate was 3 out of 17 (17.6%) cases. The median operation time was 120 min (range, 65–240 min). The highest incidence of major laparoscopic hepatectomies occurred during period 2. The median operation time was 138 min (range, 80–344 min). The conversation rate was 3 out of 27 (11.1%) cases. The median estimated blood loss was 250 mL (range, 50–2,000 mL). The first left hepatectomy was performed after 9 cases during period 2.
Postoperative outcomes and intervention were listed in Table 2. The overall postoperative morbidity was 2.3%. There was no postoperative mortality in this series. According to the Clavien-Dindo classification of post-operative complications, one Grade II complication was found in a case (urinary retention) and this was resolved by urinary catheterization. This complication had no correlation with LLR.
Discussion
Laparoscopic surgery has gradually replaced open surgery as the standard of care in many abdominal surgeries. The common advantages of LLR are shorter postoperative hospital stays, reduced estimated blood loss, lower transfusion rates, decreased postoperative morbidity and mortality, and improved cosmetic results (6,17-19). Additionally, some studies have suggested that LLR was more cost-effective than open liver resection (20). Careful selection of patients and hepatobiliary pathological diagnoses are crucial to execute LLR with safe results (21). The first international consensus conference on laparoscopic liver surgery which brought upon the “Louisville Statement” in 2008 described three techniques of LLR (total laparoscopy, hand-assisted laparoscopy and hybrid technique) and the best indication for LLR was solitary and superficial lesions of ≤5 cm situated in the peripheral liver segments (7). Furthermore, laparoscopic left lateral sectionectomy was considered as the standard surgical method for hepatobiliary diseases (7). Lately, the scope of LLR has widened to more complex procedures, from segmentectomy to hemihepatectomy (22). Based on a review of 2,804 LLR cases, the most common type of LLR was wedge resection (45%) (6), which is similar in this study (40.9%). Improvements in instruments and surgical techniques have brought about single incision laparoscopic surgery (23) and robotic surgery (24).
The development of single incision laparoscopic surgery (SILS) in minimising incisions, has been demonstrated in several surgical subspecialties (25-30). In this study, two patients with hepatic hemangioma and hepatic cystadenoma (located in the left lateral sector), underwent SILS successfully without conversion and complications. The theoretical advantages of SILS are the better conservation of collateral veins on the anterior abdominal wall and better cosmetic results (23). In addition, excessive triangulation of instruments was not needed as the parenchymal transection line is located at midline and parallel to the line of vision. Hence, it was easier to overcome the difficulty in the manipulation of instruments (31).
The introduction of robotic laparoscopic surgery in the 1990s was meant to overcome the limitations of LLR, such as the limited degrees of freedom for manipulation of instruments, tremor amplification and the adaptation of two-dimensional imaging (32). Da Vinci Surgical System (DVSS) permits three-dimensional, high-resolution binocular view of the surgical field; Endo-Wrist instruments allowing 7° of freedom for instrument movement and filtering tremor; and comfortable ergonomics for the surgeon (33). Some studies involving the usage of DVSS in LLR have been found to be safe and feasible (34,35). In this series, DVSS was employed for a wedge resection case for focal nodular hyperplasia. The demerits in using DVSS are the extremely prohibitive cost and the absence of tactile feedback (36).
Tumour location and size are two crucial elements in ascertaining the indications for LLR for patients with HCC (37). LLR with adequate resection margins is difficult to be accomplished for patients with HCC tumours located at unfavourable locations (posterosuperior) segments I, IVa, VII and VIII (38-40). Nevertheless, three studies have demonstrated that LLR can be undertaken in patients with HCC located in these segments (12,38,39). With respect to the tumour size, LLR was considered to be unfeasible for HCC larger than 10cm due to the perceived technical challenges and inadequate resection margins (6). However, one study reported that LLR is feasible in treating patients with 5 to 10 cm HCC (37). In Malaysia, HCCs are often presented at a late stage and most tumours are large-sized, located at unfavourable segments at the time of diagnosis. Small tumours located in favourable locations are predominantly treated with percutaneous radiofrequency ablation by interventional radiologists. This series described several cases of HCC and other pathologic diagnoses located at unfavourable segments and have tumour sizes larger than 10 cm, successfully underwent LRR without open conversion and complications.
The 2nd International Consensus Conference on Laparoscopic Liver Surgery ascertained the increasing popularity of LLR over the last 6 years following the “Louisville Statement” (41). Various aspects related to the laparoscopic learning curve have been examined. Most of the published literatures concentrated on one procedure or a single outcome. Vigano et al. (42) were the first to describe the learning curve of LLR based almost exclusively on CUSUM analysis over three different time periods. They concluded that the learning curve of LLR could be overcome with 60 cases. Another literature recently published by Nomi et al. suggested that 45 laparoscopic major hepatectomies were necessary to reduce the operation time and to move from the initial learning phase to the increased competence with laparoscopy phase (43). In this study, the single surgeon’s learning curve is a continuously evolving process by considering almost 10 years of experience. The learning curve was analysed with CUSUM based on the scoring system proposed by Ban et al. (8). The results depicted two different periods according to the trend in the difficulty scoring. Period 1 was regarded as the initial experience. Period 2 was thought as the period that the surgeon sought to push his boundaries due to acquired confidence and expertise by attempting to address the most challenging procedures. Therefore, the median operative time and the median estimated blood loss were higher in period 2 than in period 1. Moreover, it should be highlighted that all techniques of resection, even those located at posterosuperior segments, were included in the CUSUM analysis.
The most common motives for conversion to open resection are bleeding and poor localization of tumour (6,44). The rate of conversion to open resection was 13.6% in the present study (n=44) which was like the rates in previously reported literatures (6,45,46). Even so, conversion from LLR to open resection should not be conceived as a failure or a morbidity (3). Conversions help the surgeon to control bleeding (47). Furthermore, the morbidity rate was 2.3% and no postoperative mortality was observed.
There are little published literatures stating the number of LLR cases in the SEA region. Based on a search at PubMed, there were only less than 30 SEA literatures found at present. The first literature is a 5-year experience of LLR at a single centre in Vietnam (48). This literature described the feasibility of LLR for 173 HCC patients from January 2008 to December 2012. The other literatures were from Singapore, which stated more than 200 LLR were performed since the year 2000 (3,12,18,19,25,33-35,37).
Despite the many advantages offered by laparoscopic surgery, the uptake of LLR in Malaysia is rather slow compared to other advanced countries such as Japan or Korea in the Asia-Pacific region. This is because most of the cases of liver tumours were of large-sized at presentation, and not many liver surgeons in Malaysia are well trained in both liver surgery and advanced laparoscopic skills. Liver tumours of smaller size (<5 cm) were mostly referred by hepatologists to undergo radiofrequency ablation or trans-arterial chemoembolization (TACE). Therefore, liver surgeons in Malaysia frequently faced with challenges of large tumours or tumours located in unfavourable locations; many liver surgeons are more comfortable with the traditional open surgery. In addition, the costs of instruments and the setups of LLR are other elements that slow down the development of LLR in Malaysia. Thus, it is not easy to embark on a new program or service of LLR with restricted resources and skills. In this respect, collaboration with other HPB centres—centres that are well versed in LLR—is beneficial. Other factors that will shorten the learning curve are the standardization of procedure and the placements of ports.
This work has several limitations. Firstly, the small sample data have been retrospectively analysed, and they may be prone to recall bias. Furthermore, some of the crucial data were unavailable. Secondly, the study was limited by the single surgeon’s experience in a single centre. Hence, the findings may not be applicable to other surgeons.
In conclusion, LLR has been established as a safe and feasible alternative to open surgery in Malaysia. It is paramount that liver surgeons overcome the learning curve of LLR in a systematic stepwise approach based on personal skills and experiences, and the supports provided by institutions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Chung Yip Chan, Ser Yee Lee and Adrian Kah Heng Chiow) for the series “Advanced Laparoscopic HPB Surgery in South East Asia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.06.04). The series “Advanced Laparoscopic HPB Surgery in South East Asia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained from Sunway Medical Centre Independent Research Ethics Committee (No. 008/2018/FR). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. In: Abstracts of the 1992 Scientific Session of the Society of American Gastrointestinal Surgeons (SAGES). Washington, D.C., USA, April 11-12, 1992. Surg Endosc 1992;6:85-110. [Crossref]
- Han JH, You YK, Choi HJ, et al. Clinical advantages of single port laparoscopic hepatectomy. World J Gastroenterol 2018;24:379-86. [Crossref] [PubMed]
- Goh BKP, Chan CY, Lee SY, et al. Early experience with totally laparoscopic major hepatectomies: single institution experience with 31 consecutive cases. ANZ J Surg 2018;88:E329-33. [Crossref] [PubMed]
- Descottes B, Lachachi F, Sodji M, et al. Early experience with laparoscopic approach for solid liver tumors: initial 16 cases. Ann Surg 2000;232:641-5. [Crossref] [PubMed]
- Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 2000;232:753-62. [Crossref] [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745-53. [Crossref] [PubMed]
- Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-77. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Laparoscopic hepatectomy is theoretically better than open hepatectomy: Preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci 2014;21:723-31. [Crossref] [PubMed]
- Lee W, Han HS, Yoon YS, et al. Comparison of laparoscopic liver resection for hepatocellular carcinoma located in the posterosuperior segments or anterolateral segments: A case-matched analysis. Surgery 2016;160:1219-26. [Crossref] [PubMed]
- Teo JY, Kam JH, Chan CY, et al. Laparoscopic liver resection for posterosuperior and anterolateral lesions-a comparison experience in an Asian centre. Hepatobiliary Surg Nutr 2015;4:379-90. [PubMed]
- Clavien PA, Barkun J, De Oliveira ML, et al. The clavien-dindo classification of surgical complications: Five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Rutkauskas S, Gedrimas V, Pundzius J, et al. Clinical and anatomical basis for the classification of the structural parts of liver. Medicina 2006;42:98-106. [PubMed]
- Yap CH, Colson ME, Watters DA. Cumulative sum techniques for surgeons: A brief review. ANZ J Surg 2007;77:583-6. [Crossref] [PubMed]
- Tomassini F, Scuderi V, Colman R, et al. The single surgeon learning curve of laparoscopic liver resection. Medicine 2016;95:e5138 [Crossref] [PubMed]
- Cai XJ, Yang J, Yu H, et al. Clinical study of laparoscopic versus open hepatectomy for malignant liver tumors. Surg Endosc 2008;22:2350-6. [Crossref] [PubMed]
- Leong WQ, Ganpathi IS, Kow AW, et al. Comparative study and systematic review of laparoscopic liver resection for hepatocellular carcinoma. World J. Hepatol 2015;7:2765-73. [Crossref] [PubMed]
- Goh BKP, Chan CY, Lee SY, et al. Factors associated with and consequences of open conversion after laparoscopic distal pancreatectomy: initial experience at a single institution. ANZ J Surg 2017;87:E271-5. [Crossref] [PubMed]
- Polignano FM, Quyn AJ, de Figueiredo RS, et al. Laparoscopic versus open liver segmentectomy: Prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc 2008;22:2564-70. [Crossref] [PubMed]
- Tranchart H, Dagher I. Laparoscopic liver resection: a review. J Visc Surg 2014;151:107-15. [Crossref] [PubMed]
- Choi SB, Choi SY. Current status and future perspective of laparoscopic surgery in hepatobiliary disease. Kaohsiung J Med Sci 2016;32:281-91. [Crossref] [PubMed]
- Tayar C, Subar D, Salloum C, et al. Single incision laparoscopic hepatectomy: Advances in laparoscopic liver surgery. J Minim Access Surg 2014;10:14-7. [Crossref] [PubMed]
- Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: A matched comparison. Ann Surg 2014;259:549-55. [Crossref] [PubMed]
- Chang SK, Lee KY. Therapeutic advances: Single incision laparoscopic hepatopancreatobiliary surgery. World J Gastroenterol 2014;20:14329-37. [Crossref] [PubMed]
- Pietrasanta D, Romano N, Prosperi V, et al. Single-incision laparoscopic right colectomy for cancer: A single-centre preliminary experience. Updates Surg 2010;62:111-5. [Crossref] [PubMed]
- Takahashi T, Takeuchi H, Kawakubo H, et al. Single-incision laparoscopic surgery for partial gastrectomy in patients with a gastric submucosal tumor. Am Surg 2012;78:447-50. [PubMed]
- Surgit O. Single-incision Laparoscopic Surgery for Total Extraperitoneal Repair of Inguinal Hernias in 23 Patients. Surg Laparosc Endosc Percutan Tech 2010;20:114-8. [Crossref] [PubMed]
- Tam YH, Sihoe JD, Cheung ST, et al. Single-incision laparoscopic nephrectomy and heminephroureterectomy in young children using conventional instruments: First report of initial experience. Urology 2011;77:711-5. [Crossref] [PubMed]
- Targarona EM, Pallares JL, Balague C, et al. Single incision approach for splenic diseases: A preliminary report on a series of 8 cases. Surg Endosc 2010;24:2236-40. [Crossref] [PubMed]
- Pan M, Jiang Z, Cheng Y, et al. Single-incision laparoscopic hepatectomy for benign and malignant hepatopathy: Initial experience in 8 Chinese patients. Surg Innov 2012;19:446-51. [Crossref] [PubMed]
- Kitisin K, Packiam V, Bartlett DL, et al. A current update on the evolution of robotic liver surgery. Minerva Chir 2011;66:281-93. [PubMed]
- Goh BKP, Lee SY, Chan CY, et al. Early experience with robot-assisted laparoscopic hepatobiliary and pancreatic surgery in Singapore: single-institution experience with 20 consecutive patients. Singapore Med J 2018;59:133-8. [Crossref] [PubMed]
- Goh BK, Lee LS, Lee SY, et al. Initial experience with robotic hepatectomy in Singapore: analysis of 48 resections in 43 consecutive patients. ANZ J Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Kam JH, Goh BK, Chan CY, et al. Robotic hepatectomy: Initial experience of a single institution in Singapore. Singapore Med J 2016;57:209-14. [Crossref] [PubMed]
- Hyung WJ. Robotic surgery in gastrointestinal surgery. Korean J Gastroenterol 2007;50:256-9. [PubMed]
- Chang SK, Tay CW, Shen L, et al. Long-term oncological safety of minimally invasive hepatectomy in patients with hepatocellular carcinoma: A case-control study. Ann Acad Med Singapore 2016;45:91-7. [PubMed]
- Kim WJ, Kim KH, Shin MH, et al. Totally laparoscopic anatomical liver resection for centrally located tumors: A single center experience. Medicine 2017;96:e5560 [Crossref] [PubMed]
- Xiao L, Li JW, Zheng SG. Laparoscopic anatomical segmentectomy of liver segments VII and VIII with the hepatic veins exposed from the head side (with videos). J Surg Oncol 2016;114:752-6. [Crossref] [PubMed]
- Lee W, Woo JW, Lee JK, et al. Comparison of Learning Curves for Major and Minor Laparoscopic Liver Resection. J Laparoendosc Adv Surg Tech A 2016;26:457-64. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Vigano L, Laurent A, Tayar C, et al. The learning curve in laparoscopic liver resection: Improved feasibility and reproducibility. Ann Surg 2009;250:772-82. [Crossref] [PubMed]
- Nomi T, Fuks D, Kawaguchi Y, et al. Learning curve for laparoscopic major hepatectomy. Br J Surg 2015;102:796-804. [Crossref] [PubMed]
- Gkegkes ID, Iavazzo C. Single incision laparoscopic hepatectomy: A systematic review. J Minim Access Surg 2014;10:107-12. [Crossref] [PubMed]
- Yu X, Yu H, Fang X. The impact of body mass index on short-term surgical outcomes after laparoscopic hepatectomy, a retrospective study. BMC Anesthesiol 2016;16:29. [Crossref] [PubMed]
- Troisi RI, Montalti R, Van Limmen JG, et al. Risk factors and management of conversions to an open approach in laparoscopic liver resection: Analysis of 265 consecutive cases. HPB 2014;16:75-82. [Crossref] [PubMed]
- Nomi T, Fuks D, Govindasamy M, et al. Risk factors for complications after laparoscopic major hepatectomy. Br J Surg 2015;102:254-60. [Crossref] [PubMed]
- Long TC, Bac NH, Thuan ND, et al. Laparoscopic liver resection: 5-year experience at a single center. Surg Endosc 2014;28:796-802. [Crossref] [PubMed]
Cite this article as: Yap PY, Bong JJ. Laparoscopic liver resection in Malaysia—a single surgeon’s learning curve. Ann Laparosc Endosc Surg 2018;3:61.