Laparoscopic totally extra-peritoneal (TEP) inguinal hernia repair
Introduction
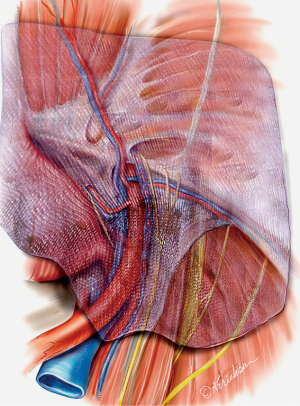
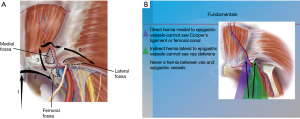
Since the initial description of laparoscopic totally extraperitoneal (TEP) inguinal hernia repair by Ferzli [1992] and McKernan [1993], abundant data have become available on laparoscopic inguinal hernia repairs and their outcomes compared to open approaches (1,2). Decreased wound complications, faster recovery, and decreased incidence of chronic pain are some of the advantages that drove more surgeons to adopt these techniques (3-5). Moreover, and with increased experience with the technique, the classic indications and applications have been extended to encompass the whole spectrum of groin hernias from the non-recurrent unilateral hernia to the more complex and recurrent cases. The indications and contraindications for TEP are summarized in Tables 1 and 2. A thorough understanding of the anatomy of the preperitoneal space is crucial when considering performing TEP (Figure 1A,B,C).
Table 1
| Indications for TEP | Reason |
|---|---|
| Recurrent hernia | Avoids scar tissue, visualizes occult hernia |
| Bilateral hernias | Decreased post-operative pain, earlier return to work |
| Groin hernias in females | Diagnose and repair concomitant femoral hernias and decrease recurrences |
| Obese patients | Definitive diagnosis, reduced infection in susceptible population |
| Patients with contralateral injury to vas deferens | Less chance to injure other vas |
TEP, totally extraperitoneal.
Table 2
| Relative contraindications | Absolute contraindications |
|---|---|
| Prior pre-peritoneal surgery (prostate, hernia, vascular, kidney transplant) | Patients for whom general anesthesia and pneumoperitoneum are risks (cardiac, pulmonary disease) |
| Prior laparotomy |
Ascites
Strangulated hernia
Giant scrotal hernia
Anticipated bleeding (patients on anti-coagulation)
TEP, totally extraperitoneal.
TEP and transabdominal pre-peritoneal (TAPP) are the two most commonly used approaches for minimally invasive inguinal hernia repair. Neither approach has been shown to be the better however several fundamental differences exist when comparing the two approaches. TEP completely avoids entry to the abdomen which can be advantageous in patients with prior surgery. TEP also avoids the issue of peritoneal closure and the problems associated with that closure. TEP can be performed without the use of electrocautery which may translate into less post-operative pain. When the space of Retzius is developed in TEP, 3/4 (femoral, obturator and direct) hernias are explored on the contralateral side. These hernias may not be readily identifiable by examining the peritoneal side.
Operative technique
Initial trocar placement
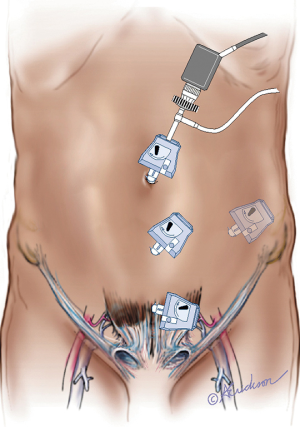
TEP requires the placement of 3 trocars in the lower midline, one Hasson and two 5 mm trocars (Figure 2) (6). A 15 mm curvilinear infra umbilical incision is made and carried down sharply to the level of the fascia. The anterior rectus sheath is incised transversely off the midline to expose the rectus abdominis muscle. Avoiding the linea alba is important to avoid inadvertent entry into the peritoneal cavity. The rectus abdominis muscle is swept laterally exposing the posterior rectus sheath. The surgeon’s index finger is inserted into the preperitoneal space and is swept from side to side to develop the space and accommodate placement of the 5 mm trocars. The two 5 mm trocars are placed guided by the index finger to prevent their placement in the intra-peritoneal position. The Hasson cannula is then placed into the space under vision with the help of S-type retractors and the pre-peritoneal space is insufflated with CO2 to 10 mmHg. A 10 mm 30-degree laparoscope is then inserted and used to further develop the preperitoneal space down to the pubic symphysis using side to side motion. The use of a balloon space maker for the initial access is an option, however it adds cost to the procedure.
The seven steps of TEP
After placement of the trocars, a stepwise approach is critical to perform a safe, effective, and reproducible repair (7). The goal is to completely dissect the myopectineal orifice (MPO) and identify all potential sites of hernia formation: (I) direct space; (II) indirect space; (III) femoral space; (IV) obturator space. Reduction of the hernia(s) follows dissection to allow finally placement of mesh and reinforcement of the whole MPO. These seven steps are summarized in Table 3 and are described in the following text as well as the attached videos (Figures 3,4) and Figure 5.
Table 3
| Steps of TEP |
| 1. Identify the pubic symphysis in the midline |
| 2. Bluntly dissect Cooper’s ligament bilaterally. This will open up the space of Retzius |
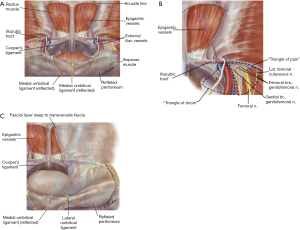
| 3. Identify Hesselbach’s triangle and the three potential sites of herniation related to it (direct, femoral, obturator) |
| 4. Identify and elevate the epigastric vessels |
| 5. Bluntly develop the space of Bogros to the level of the ASIS |
| 6. Dissection of cord structures |
| 7. Placement of mesh |
TEP, totally extraperitoneal; ASIS, anterior superior iliac spine.


Step 1
The first step is to identify the pubic symphysis in the midline. The pubic symphysis serves as an important landmark to orient the surgeon especially in complex cases. The bladder is posterior to the pubic symphysis and care must be made not to injure it when dissecting posterior to the pubic symphysis.
Step 2
Cooper’s ligament is bluntly swept clear in a medial to lateral direction while staying close to the ligament and close to the bone in a gentle and controlled manner. This will allow visualization of the femoral and obturator spaces. If a femoral or obturator hernia is encountered, they are reduced bluntly. Care must be made during reduction of femoral hernias to avoid injuring the femoral vein or the occasional corona mortis artery. Full dissection of the space of Retzius is achieved by dissecting the contralateral Cooper ligament thereby ruling out a femoral, direct and obturator hernia as well on the contralateral side.
Step 3
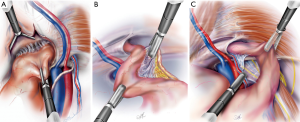
The direct space (Hesselbach’s triangle) is identified immediately superior to the femoral space and separated by the medial aspect of the iliopubic tract. A direct hernia normally obscures the view to Cooper’s ligament and is readily identifiable during the initial dissection. A convexity or a bulge in Hesselbach’s triangle signifies the presence of a large indirect hernia. A direct hernia sac is reduced by peeling it away from the attenuated transversalis fascia and using constant and gentle traction and counter-traction (Figure 6A).
Step 4
The epigastric vessels are identified and elevated anteriorly.
Step 5
While elevating the epigastric vessels, the space of Bogros is developed using horizontal blunt dissection. The space is developed laterally to the level of the anterior superior iliac spine (ASIS) to allow placement of an appropriately sized mesh.
Step 6
The indirect space is now identifiable by finding the cord structures in men or round ligament in women passing through the internal ring and lateral to the epigastric vessels. The indirect sac can be seen overlying the cord structures or round ligament. If it is not possible to identify the vas deferens or the round ligament immediately next to the epigastric vessels, then an indirect hernia is present. Prior to reduction of the indirect sac, it is important to look for and reduce cord lipomas that are found in the upper and outermost quadrant of the internal rings. Their reduction allows more working space and better identification of the edge of the indirect sac (Figure 6B). Reduction of the sac is accomplished by sweeping the cord structures or round ligament posteromedially while holding the sac superolaterally (Figure 6C).
Step 7
Numerous mesh products for inguinal hernia repair are available. Our preference is a 15 cm2 sheet of medium weight polypropylene that is trimmed to an appropriate shape and size, rolled and introduced into the space of Retzius through the Hasson port. The mesh is then unrolled and should extend from the midline to the ASIS and cover all hernia spaces (Figure 7). The mesh should be placed in the visceral plane of the extraperitoneal fascia in the space of Bogros to avoid contact with the nerves. No slit is placed in the mesh as this has been shown to be a factor in recurrences. We do not routinely tack the mesh in place. However, some may find it helpful to place a single tack in Cooper’s ligament to facilitate unrolling of the mesh. Also, and to ensure adequate medial overlap, tacking may be needed in the case of large direct and femoral hernias.
Comments/pitfalls/pearls
Pre-operative considerations
When evaluating a patient with an inguinal hernia and considering a TEP approach, several factors need to be accounted for. When taking history, questions regarding conditions that increase the likelihood of complications must be answered. Such conditions include chronic cough, constipation, and prostate problems to name a few. Modifiable risk factors such as obesity, smoking, and blood sugar control must be addressed preoperatively. The physical examination should note the size and reducibility of the hernia as well as the presence or absence of a contralateral or umbilical hernia as well as the presence or absence of nerve involvement (by looking for anesthesia, hyperesthesia or contact dysesthesia). Examination of the testicles and cord structures should be performed in men. Finally, the skin is also examined looking for rashes or the presence of fungal or bacterial infections.
Regarding patients on antiplatelet therapy or anticoagulation: Each patient should be evaluated on an individual basis, taking into account their form type of anticoagulation (anti-platelet therapy, warfarin therapy, non-VKA oral anticoagulants, heparin), duration of therapy, risk factors, and other comorbidities. Involving the patient and their treating physicians in the decision- making process would help mitigate risk and stress associated with such cases. Keeping all of those factors in mind, with experience and meticulous dissection, TEP can still be done in patients on antiplatelet therapy or anticoagulants.
Every attempt must be made to reduce an inguinal hernia on the operative table after induction of anesthesia. If successful, this simple maneuver will make the operation much easier.
Prior operative reports must be obtained in cases of recurrent hernias looking for the presence and type of mesh, plugs, or tacks and the intra-operative findings.
There are no data to support routine pre-operative antibiotic prophylaxis for laparoscopic inguinal hernia repair.
In TEP, the umbilicus-pubis distance and panniculus thickness are critical for trocar placement such that in obese patients with a thick pannus and a lower umbilicus, inadequate placement of the trocars can lead to a decreased working space and excessive torque (see Figure 8). Flexing the operating table can help augment the umbilicus-pubic distance and widen the operative field.

Having the patient empty their bladder pre-operatively spares the patient a Foley catheter placement in routine cases. However, in complex cases, such as giant scrotal hernias, Foley catheters are useful and can help diagnose bladder injuries. When a Foley catheter is placed and a bladder injury exists, the urine collection bag will insufflate with CO2.
Although most of the time TEP is performed under general anesthesia with muscle relaxation, it could be performed under local, regional (epidural) or general anesthesia without muscle relaxants and use of a laryngeal mask airway if needed (10,11).
TEP could also be performed with 2 mm instruments also known as needlescopic surgery (12).
Technical considerations
On rare occasions, and when using blunt finger dissection to develop the preperitoneal space, the finger will not cross the midline as a result of a thick linea alba extending down to the pubic symphysis. To overcome this situation, the contralateral anterior rectus sheath is also incised transversely. S-type retractors are inserted on each side of the linea alba, lifting the left and right anterior rectus sheaths. The view will be that of an inverted V similar to the vocal cords. The left and right insertions of the posterior rectus sheaths are divided to connect the left and right retromuscular compartments to the preperitoneal space (Figure 9).

When reducing an indirect sac and separating it from the cord structures, the dissection should point towards the distal end of the sac as it tapers close to the internal ring. Should inadvertent tearing of the peritoneum occur, it is easier to fix in that location after complete sac mobilization. The tear can be closed with an endoloop or clips. We do not recommend Veress needle decompression if pneumoperitoneum occurs as the space does not collapse if adequately dissected following the seven described steps. The space of Retzius is especially resistant to collapse when widely dissected and is used as the initial working space for mesh placement.
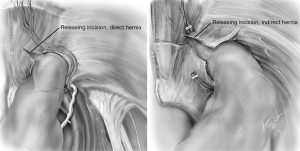
When dealing with complex and incarcerated hernias a few extra steps may prove helpful. Insertion of an additional 4th 5 mm trocar may be needed to facilitate the exposure (13,14) (Figure 1). The epigastric vessels can be divided to facilitate the dissection of the sac and to prevent the wrapping of the mesh. In the case of large and incarcerated hernias, the transversalis sling is divided with hook cautery at the 10 o’clock position to allow complete reduction of the sac (Figure 10). If a testicle and tunica vaginalis are present in the space, it is preferable to divide the sac rather than reduce it to minimize de-vascularization. If an incarcerated hernia is not able to be reduced despite relaxing incisions and adequate dissection of the preperitoneal space, a hybrid approach can be done (15). A counter incision is made and the hernia is reduced in a standard open fashion. The mesh is then unrolled in the pre-peritoneal space.
Routine mesh fixation is not recommended due to concerns of acute and chronic pain without a decrease in hernia recurrence (16,17). Should mesh fixation prove necessary such as in the case of large direct defects, mesh fixation should be done in 3 locations and preferably with an absorbable tacker. The 3 locations are: pubic symphysis, Cooper’s ligament, and anterior abdominal wall lateral to the epigastric to avoid the triangle of pain and triangle of doom.
In the preperitoneal space, deep to the transversalis fascia, is a fascial layer analogous to Scarpa’s fascia referred to as the spermatic fascia or extra-peritoneal fascia (Figure 1C). The inferior epigastric, the iliac vessels, the nerves are found external to this layer. Dissection should be carried in the plane between the peritoneum and the spermatic fascia. This signifies that as the peritoneum is peeled off the epigastric vessels, there will still be a thin translucent layer of tissue covering them. Leaving this thin layer intact will provide a barrier between the mesh and important structures such as nerves and the vas deferens and help reduce the chance of ischemic orchitis, infertility, and chronic pain (7).
Special consideration: TEP after TEP or TAPP
TEP after TEP or TAPP is a challenging procedure requiring proficient knowledge in anatomy and meticulous dissection and can be performed if an open anterior approach has also failed (18). The challenges arise from adhesions, leading to obscuring of normal anatomical landmarks and loss of working space with difficulty in developing the spaces of Retzius and Bogros. The key features of a TEP after TEP or TAPP are as follows:
- Development of the working space should be done in a plane between the old mesh and the anterior abdominal wall to keep the peritoneum intact.
- Identification of the epigastric vessels will lead to the identification of the hernia. Hernias do not normally have adhesions, and subsequently the presence of dense adhesions means that there is probably no hernia.
- Ligation of the epigastric vessels or their branches is done routinely to achieve adequate hemostasis because bleeding will compromise the exposure.
- Dissection of the hernia sac is done sharply without electrocautery whereas in primary hernias most dissection is blunt with traction and counter traction.
- External palpation and pulling of the testicle will aid in the identification of the cord structures.
- Although the working space is limited and may only allow placement of a smaller sized mesh, every attempt must be made to place a large mesh. Failing to place a large mesh increases recurrence rates and in that case, an anterior approach is preferred.
Post-operative care
Application of ice packs and administration of non-steroid anti-inflammatory drugs will help decrease the swelling in addition to providing analgesia without increasing bleeding.
Conclusions
Modus operandi, mastery of the anatomy, and meticulous direction are the 3 M’s that need to be remembered in order to perform TEP with reproducibility, safety, efficiency and good outcomes. Mastery of the anatomy of the versatile pre-peritoneal space allowed surgeons to become facile in operations such as prostatectomies and retroperitoneal node dissections (19). When performed sequentially and followed religiously, the seven steps described here allow for improved outcomes and serve as a good teaching base for surgeons training to perform TEP.
Acknowledgments
All illustrations are courtesy of Anne Erickson CMI.
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.03.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images and videos.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferzli GS, Massad A, Albert P. Extraperitoneal endoscopic inguinal hernia repair. J Laparoendosc Surg 1992;2:281-6. [Crossref] [PubMed]
- McKernan JB, Laws HL. Laparoscopic repair of inguinal hernias using a totally extraperitoneal prosthetic approach. Surg Endosc 1993;7:26-8. [Crossref] [PubMed]
- Westin L, Wollert S, Ljungdahl M, et al. Less Pain 1 Year After Total Extra-peritoneal Repair Compared With Lichtenstein Using Local Anesthesia: Data From a Randomized Controlled Clinical Trial. Ann Surg 2016;263:240-3. [Crossref] [PubMed]
- Bittner R, Arregui ME, Bisgaard T, et al. Guidelines for laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal hernia Surg Endosc 2011;25:2773-843. [International Endohernia Society (IEHS)]. [Crossref] [PubMed]
- Bittner R, Montgomery MA, Arregui E, et al. Update of guidelines on laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal hernia (International Endohernia Society). Surg Endosc 2015;29:289-321. [Crossref] [PubMed]
- Ferzli GS, Fingerhut A. Trocar placement for laparoscopic abdominal procedures: a simple standardized method. J Am Coll Surg 2004;198:163-73. [Crossref] [PubMed]
- Ferzli, GS, Edwards ED. Laparoscopic Inguinal Herniorrhaphy. In: Cameron JL, Cameron AM. editors. Current Surgical Therapy. 10th Edition. Philadelphia, PA: Elsevier, 2011:1197-210.
- Ferzli G, Iskandar M. Laparoscopic totally extraperitoneal (TEP) repair of right inguinal hernia. Asvide 2019;6:080. Available online: http://www.asvide.com/article/view/30691
- Ferzli G, Iskandar M. Totally extraperitoneal (TEP) for right direct inguinal hernia. Asvide 2019;6:081. Available online: http://www.asvide.com/article/view/30692
- Ferzli G, Sayad P, Vasisht B. The feasibility of laparoscopic extraperitoneal hernia repair under local anesthesia. Surg Endosc 1999;13:588-90. [Crossref] [PubMed]
- Lal P, Philips P, Saxena KN, et al. Laparoscopic total extraperitoneal (TEP) inguinal hernia repair under epidural anesthesia: a detailed evaluation. Surg Endosc 2007;21:595-601. [Crossref] [PubMed]
- Ferzli G, Sayad P, Nabagiez J. Needlescopic extraperitoneal repair of inguinal hernias. Surg Endosc 1999;13:822-3. [Crossref] [PubMed]
- Ferzli GS, Kiel T. The role of the endoscopic extraperitoneal approach in large inguinal scrotal hernias. Surg Endosc 1997;11:299-302. [Crossref] [PubMed]
- Ferzli G, Shapiro K, Chaudry G, Patel S. Laparoscopic extraperitoneal approach to acutely incarcerated inguinal hernia. Surg Endosc 2004;18:228-31. [Crossref] [PubMed]
- Ferzli GS, Rim S, Edwards ED. Combined laparoscopic and open extraperitoneal approach to scrotal hernias. Hernia 2013;17:223-8. [Crossref] [PubMed]
- Ferzli GS, Edwards E, Al-Khoury G, et al. Postherniorrhaphy groin pain and how to avoid it. Surg Clin North Am 2008;88:203-16. x-xi. [Crossref] [PubMed]
- Ferzli GS, Edwards ED, Khoury GE. Chronic pain after inguinal herniorrhaphy. J Am Coll Surg 2007;205:333-41. [Crossref] [PubMed]
- Ferzli GS, Khoury GE. Treating recurrence after a totally extraperitoneal approach. Hernia 2006;10:341-6. [Crossref] [PubMed]
- Sayad P, Ferzli G. The extraperitoneal approach and its utility. Surg Endosc 1999;13:1168-9. [Crossref] [PubMed]
Cite this article as: Ferzli G, Iskandar M. Laparoscopic totally extra-peritoneal (TEP) inguinal hernia repair. Ann Laparosc Endosc Surg 2019;4:35.