
Endoscopy in surgically altered anatomy
Introduction
Having a solid understanding of patient anatomy is paramount to performing any procedure. The ability to intubate the esophagus with an endoscope would not be possible without knowing the position and trajectory in relation to adjacent structures such as the trachea. Centuries of anatomy research have established that core knowledge of basic human anatomy. The challenge of modern endoscopy is understanding anatomy that has been surgically altered. As surgical therapies expand, more patients will present to the endoscopy suite with altered gastrointestinal anatomy.
As previously stated, a firm understanding of the anatomy is key to performing any procedure. If unfamiliar with basic principles of specific surgical procedures, a quick literature or online search can provide pictures and diagrams of the surgical anatomy. Additionally, discussion with the original surgeon can provide invaluable information regarding surgeon-specific preferences or methods such as orientation of the Baker’s pouch in Roux-en-Y gastric bypass (RYGB). This discussion can be especially beneficial as operative reports do not always effectively reflect the critical details of a procedure and may be lacking important details (1-3). All these resources can provide critical information to assist the endoscopist in identifying pathology.
The second stage of successful endoscopy is accurately documenting findings to provide a roadmap for other clinicians. Current endoscopy software programs are focused on standard diagnostic procedures in the setting of native anatomy. Unfortunately, adapting standard definitions to nonstandard anatomy can create confusion rather than resolve it. In these situations, free text entries and photos or video documentation are essential to bridge the gap and allow accurate transmission of information.
Combining an accurate anatomic understanding with the ability to effectively communicate findings makes the endoscopist an irreplaceable member of the treatment team. In this article we describe some of the common surgical procedures that result in altered gastrointestinal anatomy and suggest methods for preprocedural planning, procedural tips, treatment options, and essential documentation to aid future discussion and direct clinical care.
Bariatric
Post-bariatric surgery is probably the most common surgically-altered anatomy encountered by endoscopists. Surgical weight loss has evolved since first being introduced in the 1960s (4). RYGB, adjustable gastric band, sleeve gastrectomy, and several variations have all been utilized with some frequency to treat morbid obesity. Because of this variability, a sound fundamental knowledge of the different surgical techniques is essential to diagnosing and treating any postoperative complications.
Roux-en-Y gastric bypass was one of the original surgical weight loss procedures but was only used in select cases due to overall patient morbidity. With the introduction of laparoscopic techniques, operative risk improved and RYGB was available to a broader patient population. Roughly 320,000 patients underwent RYGB in the United States between 2011 and 2016 and it was the second most common bariatric procedure performed globally in 2016 (5,6). The technique of RYGB includes stapling the proximal stomach to create a small gastric pouch along the lesser curvature. The jejunum is transected approximately 40 cm distal to the ligament of Treitz and a gastrojejunal (GJ) anastomosis is created between the distal bowel and the gastric pouch in an end-to-side fashion. This end-to-side anastomosis leaves a blind end (often called Baker’s pouch) adjacent to the alimentary limb. Distally, intestinal continuity is restored by creating a side-to-side jejunojejunal anastomosis (7) (Figure 1).
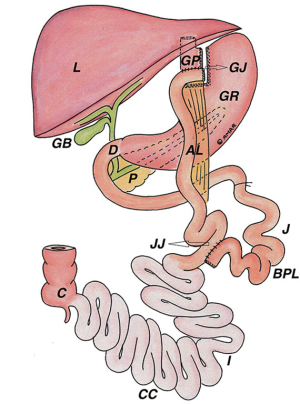
Upper endoscopy in a post-RYGB patient should reveal a relatively small gastric pouch measuring roughly 40 cc. The GJ anastomosis should be <2 cm in diameter (Figure 2). Marginal ulceration is a common problem in bypass patients and careful inspection of the anastomosis is crucial to initiate treatment if found. Passing through the anastomosis reveals two limbs. Depending on surgeon preference, the alimentary limb may be on the patient’s right or left. The blind limb should be inspected for retained food or excessive length which may cause symptoms of epigastric discomfort or food intolerance. Traversing the alimentary limb should reveal normal appearing mucosa. Bile may be present particularly as you approach the jejunojejunal anastomosis. Standard alimentary limb length is 75–100 cm usually permitting visualization of the jejunojejunal anastomosis with a diagnostic gastroscope. If the jejenojejunal anastomosis requires surveillance and you are unable to reach it with a standard length gastroscope, a colonoscope may provide the additional length required to evaluate the anastomosis. Push enteroscopy or balloon-assisted endoscopy are additional options. The common channel is identified by following the plicae circularis which will be continuous with the alimentary limb while the biliopancreatic limb will be truncated. Procedural variation can alter these standard findings and careful review of operative notes or discussion with the surgeon can provide crucial information.
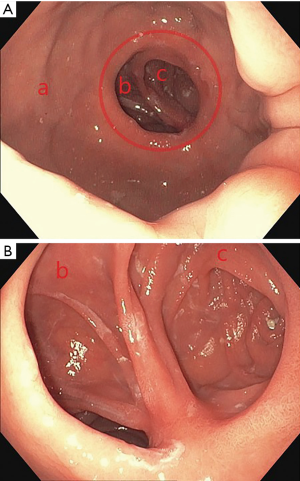
Upper endoscopy in post-RYGB patients can be utilized to assess early postoperative complications and late postoperative complaints such as epigastric pain, reflux, and weight regain (8).
Weight regain: weight regain is a common late concern in the post-RYGB patient. It is reported that most bariatric patients regain 10–15% percent of their total body weight loss in the years following surgery (9). A subset of patients regain significantly more weight and some even gain back to their pre-surgical weight. Undesirable weight regain after RYGB can be the result of the patient resuming maladaptive diet habits or technical factors associated with the procedure—specifically dilation of the gastric pouch or GJ anastomosis. Patients may report lacking the same early satiety initially felt after the surgery. They may describe the ability to consume normal portion sizes without restriction. These are indications for diagnostic upper endoscopy for evaluation of the pouch and anastomosis to determine the cause.
A typical RYGB gastric pouch should not permit scope retroflexion. Additionally, evidence of a large retained fundus is an indicator that the pouch is oversized. The GJ anastomosis is created in a handsewn or stapled fashion but should be less than 20 mm in diameter. Measuring the pouch or the GJ anastomosis can be performed using a fully expanded 30 mm endoscopic snare to create scale. If the pouch or anastomosis is oversized, this may be a cause for the patient’s weight regain and may lead the surgeon to discuss options for surgical correction with the patient.
A third cause of weight regain after RYGB is the development of a gastrogastric (GG) fistula between the pouch and remnant stomach. This is typically diagnosed with contrast-enhanced upper radiographs that show contrast entering the remnant stomach on initial images. Upper endoscopy is beneficial to identify and potentially treat the GG fistula. The superior staple line of the pouch is the most likely culprit as it is the most difficult to completely transect at the initial operation and is in closest proximity to the pouch after transection. This can result in delayed reconstitution of gastric continuity. To identify the fistula on upper endoscopy, the staple line should be interrogated looking for any pits, irregularities, or erythema. Direct visualization, catheter probe, direct contrast injection with fluoroscopy, or a combination of these are the best methods of endoscopic identification. A distal cap attachment can help deflect rugal folds and interrogate the mucosa.
Epigastric pain after gastric bypass is a common compliant and includes pathology such as ulceration, bile reflux, and pouch gastritis. Marginal ulceration at the GJ anastomosis and pouch gastritis require maximal acid suppression and repeat interval endoscopy to ensure appropriate treatment and healing to prevent full thickness perforation. Evidence of bile in the alimentary limb and gastric pouch as well as evidence of gastritis are indicative of bile gastritis and respond to mucosal protection strategies such as sucralfate. These findings are essential to communicate back to the operating surgeon for ongoing treatment and follow-up.
Gastric access: percutaneous enteral access after gastric bypass is challenging but may be required for the treatment of chronic malnutrition, medication administration, or for enteral decompression. Options for access, depending on indication, include gastrostomy tube placement into the gastric remnant, gastric pouch, or the alimentary limb. Access to the gastric pouch for endoscopic or percutaneous tube placement is challenging because of the small size and typical location of the pouch above the xiphoid process and costal margin (10). When no safe window exists for direct access to the gastric pouch, a second option is to perform a percutaneous endoscopic jejunostomy tube into the alimentary limb. When performing this procedure, it is important to note whether the alimentary limb lies in an antecolic or retrocolic position as indicated in the operative records. If the alimentary limb is retrocolic, endoscopic jejunostomy tube placement is not advised due to the overlying colon and alternative access would be indicated. The gastric remnant is a third suitable site for enteral access and is often needed to treat chronic distension of the remnant or to provide stable access to the biliary tree. Access to the gastric remnant can be obtained through balloon assisted endoscopy and PEG tube placement into the gastric remnant or case reports describe endoscopic ultrasound techniques to directly puncture the gastric remnant through the gastric pouch (11). Patients with a long alimentary limb may require alternative modes of access such as radiologic-guided percutaneous access to the gastric remnant (12).
Sleeve gastrectomy is the current gold standard surgical weight loss operation performed in North America because of weight loss results and health benefits similar to RYGB but without the long-term complications of RYGB (13). In 2016 it was the most commonly performed bariatric procedure worldwide (6,13). Sleeve gastrectomy is performed by transecting the stomach with sequential firings of a surgical stapler beginning approximately 6 cm from the pylorus and continuing up to the angle of His. Sizing the sleeve is performed over a bougie or endoscope to create a roughly 2 cm wide conduit and provide the restrictive component of the operation that promotes weight loss (Figure 3).
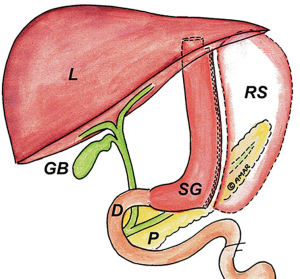
Patients may be referred to endoscopy for acute postoperative complications or for long-term complaints such as epigastric pain and weight regain. Acute complications such as staple line leak may require endoscopic evaluation and intervention. Like RYGB, deep pits or pockets, erythema, or purulence along the staple line may help localize the source of leak. Evaluating the staple line with a distal cap attachment and on table contrast fluoroscopy can aid in identifying the source of leak.
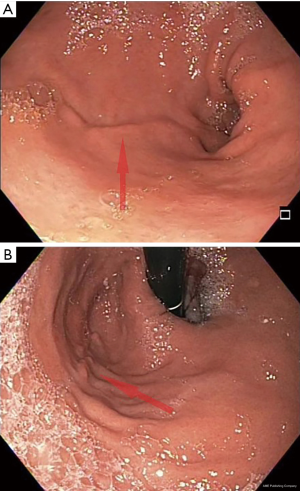
Outside of the acute setting, endoscopy should reveal a normal appearing gastroesophageal (GE) junction without evidence of hiatal hernia. A healed staple line, appearing as a mucosal ridge, will run as a continuous line from the incisura to the angle of His on the patient’s left. The staple line should be straight, and the endoscope should pass easily through the sleeve into the antrum (Figure 4). A spiraling staple line or tight angulation at the level of the incisura may indicate a twisted sleeve and results in symptoms similar to gastric outlet obstruction. A small portion of proximal fundus may be present above the staple line but should not permit retroflexion. A large retained fundus may be a cause of weight regain if the patient reports no longer having the sensation of early satiety and restriction. Surgery is required to remove this portion of retained fundus.
Adjustable gastric banding is like vertical banded gastroplasty in that it creates a small gastric pouch by placing a band around the upper portion of the stomach as an external constrictor. The band can be inflated or deflated through tubing connected to a small port placed in the patient’s abdominal wall. Gastric bands were initially very common because it could be performed as an outpatient procedure. Long-term complications and inconsistent weight loss led most surgeons to no longer offer this operation. Complications of the procedure include band erosion, slipped band, and intolerance. Patients may present with epigastric pain, chest pain, dysphagia, and regurgitation.
The first step in managing these patients is to deflate the band followed by an upper GI swallow study. It is important to note that patients with a gastric band represent an aspiration risk even after the band has been deflated. On endoscopy a small gastric pouch should be visualized without significant erythema or edema. A standard gastroscope should pass through the gastric outlet without significant pressure. Difficulty passing the scope may indicate a slipped band or early band erosion and edema. Passing through the gastric outlet, the endoscope should be retroflexed to evaluate the band position in a retrograde fashion. The mucosa should be inspected for evidence of erythema or irritation which may indicate impending band erosion. Visualizing the band material indicates the band has eroded either partially or completely through the gastric wall and requires surgical removal of the band system and revisional surgery (Figure 5).
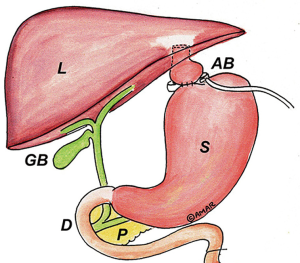
Biliopancreatic Diversion with Duodenal Switch (BPDS) results in the greatest excess body weight loss but has the highest morbidity and mortality of the surgical weight loss procedures (14). Sleeve gastrectomy is the first stage of BPDS to create the restrictive component of the operation. The second stage includes dividing the stomach at the pylorus and the jejunum in the midportion of the bowel. The distal jejunum is then mobilized to create a new gastro-jejunostomy and intestinal continuity is reestablished in a Roux-en-Y configuration (15) (Figure 6). Upper endoscopy in patients with BPDS demonstrates a gastric sleeve with a GJ anastomosis. The jejunal mucosal has a different appearance than duodenal mucosa. Like in RYGB, marginal ulceration at the GJ anastomosis is possible and circumferential evaluation of the anastomosis should be performed (16).
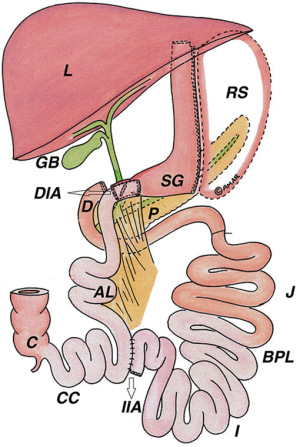
Vertical banded gastroplasty is no longer considered a standard surgical weight loss option, but patients with a history of vertical banded gastroplasty may still present to the hospital or your clinic for evaluation. The operation is performed by creating a small proximal pouch using a surgical stapler without completely transecting the stomach thereby maintaining normal gastric intestinal continuity. A gastric band is placed around the medial portion of the stomach through a circumferentially stapled gastrotomy to create a small pouch (Figure 7). The type of band used ranged from commercially-available products to surgeon-fashioned devices made from IV tubing, suture material, or mesh. Complications following vertical banding gastroplasty were common and lead to the cessation of the procedure being performed. These complications included band erosion, staple line breakdown, and GG fistula formation (17).
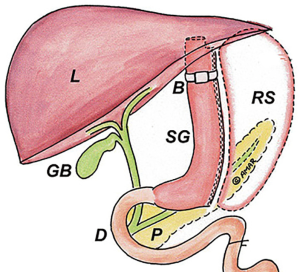
Endoscopy in a patient with a vertical banded gastroplasty would demonstrate a small proximal gastric pouch with a left lateral staple line and single lumen. The outlet restriction to the pouch is created by the band. Significant swelling may cause the lumen to be narrow and require a slim scope to traverse. Band erosion typically occurs on the distal side of the outlet and requires retroflexion in the distal stomach to evaluate for any evidence of band erosion. Significant erythema and edema or visualizing any portion of the band within the stomach is an indication for surgical removal and revisional bariatric surgery to avoid complete erosion or perforation. GG fistula formation is a source of weight regain and abdominal complaints. Like RYGB, evaluation is improved with on table fluoroscopy and interrogation of the entire staple line.
Gastrectomy
Indications for gastrectomy include peptic ulcer disease, malignancy, and rarely caustic ingestions. The specific indication determines the extent of resection—wedge, partial, or total gastrectomy. The alteration in gastrointestinal anatomy is important when performing upper endoscopy on these patients.
Wedge resection is indicated in the setting of small tumors along the greater curvature of the stomach—typically gastrointestinal stromal tumors and other benign lesions requiring tissue diagnosis (18). Wedge resection is performed using multiple fires of a surgical stapler to resect a triangulated wedge of stomach to include the lesion. The lesion must be far enough away from the GE junction, angle of His, and pylorus to be amendable to wedge resection. Postoperative endoscopic surveillance is common in these patients—particularly in the setting of a resected gastrointestinal stromal tumor. The staple line should be surveilled for any evidence of recurrence and biopsies taken as suspected. In the acute setting, endoscopy may be utilized to identify a staple line leak. Gentle interrogation of the staple line with a distal cap attachment, flexible tip wire, or contrast injection catheter with on-table fluoroscopy can aid in the diagnosis. Therapeutic endoscopic instruments can often be used to manage leaks in this situation.
Partial or subtotal gastrectomy: peptic ulcer disease was once a common indication for partial gastrectomy in the setting of refractory ulcers. Improvements in medical therapy, the discovery of Helicobacter pylori, and subsequent treatment have largely rendered peptic ulcers a medically treatable disease. Partial or subtotal gastrectomy or more commonly performed for small, low-grade gastric cancers in modern practice (19) (Figure 8). After resection, reestablishing gastrointestinal continuity can be performed in one of 3 ways.
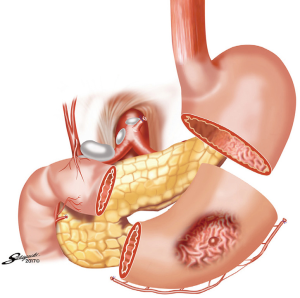
Billroth I was one of the first standard operations for the treatment of antral gastritis and peptic ulcer disease. A resection of the gastric antrum and pylorus was performed to include the ulcer or lesion. The duodenum was then mobilized along its lateral attachments and a primary gastroduodenal anastomosis is performed. Complications of this procedure included bile reflux and often required conversion to a Roux-en-Y type reconstruction. Because of this, Billroth I reconstruction was largely replaced with the reconstructive operations detailed next. In a patient who has had a prior Billroth I reconstruction it is important to note that they have significant refluxed bile in the stomach and endotracheal intubation should be strongly considered for endoscopy (20).
Billroth II was the second-generation operation devised for reconstruction after distal gastrectomy. Formal resection was performed of the gastric antrum and peptic ulcer or lesion. The duodenal stump was left in situ and reconstruction was performed by mobilizing a loop of jejunum and performing an end-to-side gastrojejunostomy. This second-generation operation was developed to alleviate the alkaline reflux side effect of the original operation. Upper endoscopy will demonstrate a foreshortened stomach with a distal GJ anastomosis with 2 lumens. One limb of the anastomosis will lead to the biliopancreatic limb and the other will lead to the alimentary limb. Patients with Billroth II reconstruction are prone to marginal ulceration similar to Roux-en-Y reconstruction, but are also prone to afferent loop syndrome which is characterized by bowel stasis and dilation of the biliopancreatic limb (21). Treatment of afferent loop syndrome is surgical and requires conversion to a Roux-en-Y or creation of a Braun enterostomy to promote drainage and antegrade flow of bile (22).
Roux-en-Y reconstruction for subtotal or partial gastrectomy has the same configuration as when performed for surgical weight loss and only differs by the size of the pouch and length of the alimentary limb. This is beneficial when trying to access the biliary tree as the shorter alimentary and biliopancreatic limb are more likely to be amendable to retrograde access. Patients with Roux-en-Y reconstruction require ongoing oncologic surveillance and are at risk for marginal ulceration and other acute complications of their surgery similar to gastric bypass for weight loss. Endoscopic surveillance is performed in standard fashion.
Total gastrectomy is often required for gastric adenocarcinoma because of its aggressive submucosal spread. Reconstruction after total gastrectomy is performed in a Roux-en-Y configuration. The esophagojejunal anastomosis may be handsewn or performed with the circular stapler depending on the surgeon’s preference and other operative factors. Patients with total gastrectomy and esophagojejunal anastomosis represent an aspiration risk due to the lack of normal anatomic antireflux mechanisms. During induction of anesthesia appropriate precautions are necessary and endotracheal intubation strongly considered (20).
Pancreaticoduodenectomy (Whipple procedure)
Pancreaticoduodenectomy, or Whipple procedure, is performed for benign and malignant lesions near the second portion of the duodenum including lesions in the pancreas, bile ducts, or duodenum. Because of the complex anatomy, en bloc resection is required at the confluence of major structures followed by reconstruction. Reconstruction needs to account for drainage of the pancreatic duct, common bile duct, and stomach. A mobilized blind limb of jejunum is the standard conduit to create a pancreaticojejunostomy and hepaticojejunostomy to drain the pancreas and bile ducts respectively. Restoration of gastrointestinal continuity is performed with either a Roux-en-Y or Billroth II reconstruction as discussed previously (23,24).
Complications after pancreaticoduodenectomy that require endoscopic treatment include strictures and leaks occurring at any of the three anastomoses created for the operation. While the gastro-jejunostomy is easy to access, the hepaticojejunostomy and pancreaticojejunostomy may be difficult to access if the patient has undergone a Roux-en-Y bypass as opposed to a Billroth II. In a Roux-en-Y bypass, the length of the alimentary and biliopancreatic limb may prove too long and tortuous to access with a standard gastroscope. In such cases a standard colonoscope may provide sufficient length or balloon-assisted endoscopy may be required to reach the biliopancreatic anastomoses (25).
Esophagectomy
Esophagectomy is performed for benign and malignant conditions of the esophagus. Reconstruction of the gastrointestinal tract requires a gastric, jejunal, or colonic conduit to span the gap (26). A gastric conduit is the most common interposition graft because it carries the native blood supply and requires a single anastomosis. The stomach is tubularized along the blood supply of the greater curvature to create an approximate 5cm conduit and a pyloromyotomy is performed to promote drainage. In direct contrast to the gastric conduit for esophageal replacement, a sleeve gastrectomy is created by stapling parallel to the lesser curvature. The staple line therefore is found on the left in a sleeve gastrectomy and to the right in a gastric conduit. This is important to note when performing endoscopic procedures for leak or staple line complications. When surveilling the gastric conduit, the staple line should be relatively straight and not spiraling which could indicate a twisted conduit and may impede gastric emptying (27).
Esophagectomy with reconstruction is performed in a transthoracic (Ivor-Lewis) or transhiatal fashion. The difference between the two procedures is the location of the esophagogastric anastomosis. In a transthoracic approach, the anastomosis is created at the level of the thoracic esophagus. This is important because a staple line leak at this level can rapidly lead to mediastinitis and sepsis but is also amendable to placement of a covered stent in certain circumstances (28). In a transhiatal esophagectomy, the esophagogastric anastomosis is placed at the level of the cervical esophagus. While a leak at this level can typically be drained through the neck, esophageal stenting at this level is typically not well tolerated because of the location near the cricopharynx.
Fundoplication
Fundoplication is performed for medically refractory GE reflux disease (29). The operation is performed by mobilizing the gastric fundus which is then circumferentially or partially wrapped around the GE junction to augment lower esophageal sphincter pressure and prevent GE reflux. Expected endoscopic findings after successful fundoplication include the GE junction positioned at the level of the hiatus and a characteristic “stack of coins” appearance of the folded mucosa with retroflexion in the body of the stomach (Figure 9).
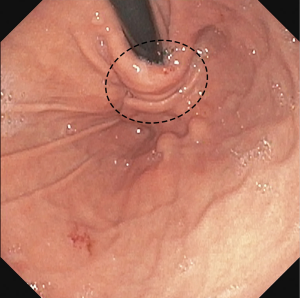
Common postoperative complications requiring upper endoscopy include dysphagia, concern for leak, and recurrent reflux symptoms. Upper endoscopy typically follows contrast-enhanced upper gastrointestinal radiographs which can provide valuable initial information regarding anatomy and function. Causes of postoperative dysphagia include normal postoperative swelling, slipped fundoplication, or herniated wrap. In those cases, the GE junction will be narrow and difficult to traverse. Postoperative swelling typically presents in the early postoperative period, but the wrap and GE junction remain below the level of the diaphragm. A slipped fundoplication occurs when the gastric fundus herniates above the wrap and is evidenced by the GE junction and a portion of dilated stomach protruding above the wrap. A herniated fundoplication demonstrates the entire GE junction and wrap above the crural pinch. A disrupted fundoplication occurs when the external gastric sutures fail causing the wrap to unfold. The characteristic “stack of coins” seen on retroflexion is absent in a disrupted fundoplication. In the case of postoperative swelling, early dysphagia gradually resolves and requires no intervention whereas the slipped, herniated, and disrupted fundoplication may require reoperation (30).
Lower gastrointestinal
Colon and rectal resections are performed for a variety of indications including infection, malignancy, and benign conditions. After resection, reestablishing intestinal continuity is performed in a variety of ways or not at all in the setting of an end ostomy. The type of anastomosis performed depends on the type of operation required. The options for anastomosis include end-to-end, side-to-end, and side-to-side configuration and the method of creating the anastomosis may be handsewn or stapled in a linear or circular fashion (31). Each of these configurations will have a different intraluminal appearance and are prone to different complications and pathology.
Colonic resections performed for diverticular disease or malignancies can be reconstructed with a single ileocolonic or colocolonic anastomosis whereas total abdominal colectomies are reconstructed with complex ileoanal anastomosis (Figure 10). An end-to-end anastomosis is performed in a handsewn fashion or with the use of a circular stapler. Endoscopically the anastomosis appears as a single concentric ring of normal caliber bowel with surgical staples or suture potentially visible. End-to-end anastomoses are more commonly used in the low pelvis where working space is limited and there is less redundancy in the bowel. Circular stapled or handsewn end-to-end anastomoses are specifically prone to narrowing or stricture as the size of the anastomosis can only be as large as the caliber of the bowel. This differs distinctly form a side-to-side anastomosis which can create a larger aperture along the antimesenteric border of the bowel. When performed in a stapled fashion, common stapler lengths are 60–90 mm in length whereas circular staplers are less than 35 mm in diameter. A relatively fresh side-to-side anastomosis will appear as two loops of bowel with plicae circularis in parallel and is prone to ischemia and leak along the antimesenteric border of the anastomosis. Conversely, an older side-to-side anastomosis appears patulous and redundant. Alternatively, a side-to-end anastomosis can be created between the proximal and distal bowel respectively. A circular firing stapler is inserted into each segment of the bowel to create a common channel. Endoscopically the anastomosis will appear as a single concentric ring. However, passing through the anastomosis will demonstrate two lumens. One end will be a short blind ending limb while the other is the proximal bowel.
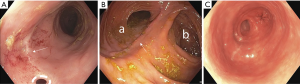
Endoscopy plays an increasingly important role in the management of complications following lower gastrointestinal surgery (32,33). Bleeding, leak, and stricture are all complications of the operation focused around the anastomosis and the incidence of each vary depending on the type of anastomosis created. Important considerations when performing endoscopy include the location of the anastomosis (distance from anus), and the type of anastomosis performed. The location will dictate what type of endoscope is necessary to reach the anastomosis. The type of anastomosis helps determine where problems are likely to occur.
Conclusions
As the instrumentation and functionality of gastrointestinal endoscopy evolves, the role in the diagnosis and management of postsurgical pathology expands. The functionality, however, is contingent on the ability to accurately obtain and convey information. A comprehensive understanding of the most common post-surgical anatomy is essential and is hopefully aided by the information detailed in this manuscript. Additional information can be obtained through direct communication with the operating surgeon, review of the operative dictation, and review of the literature. Conveying the information is best done through narrative and pictures rather than relying on standard definitions typically included in endoscopic documentation software. Failure to accurately communicate findings undermines the work done and can drastically alter patient treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery for the series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery”. The article has undergone external peer review.
Conflicts of Interest: The series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” was commissioned by the editorial office without any funding or sponsorship. RJ served as the unpaid Guest Editor of the series. EP receives honoraria and research support from Cook Biotech, Inc. and C.R. Bard, Inc., consultant fees from Boston Scientific Corp. and Actuated Medical, Inc., and royalties from UpToDate, Inc. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Novitsky YW, Sing RF, Kercher KW, et al. Prospective, blinded evaluation of accuracy of operative reports dictated by surgical residents. Am Surg 2005;71:627-31; discussion 631-2. [PubMed]
- Stogryn S, Hardy K, Vergis A. Bariatric operative reporting: quality assessment and perceptions among bariatric surgeons. Surg Obes Relat Dis 2017;13:429-35. [Crossref] [PubMed]
- Vergis A, Stogryn SE, Mullan MJ, et al. Electronic synoptic reporting: assessing the completeness of synoptic and narrative reports for Roux-en-Y gastric bypass. Surg Obes Relat Dis 2017;13:1863-8. [Crossref] [PubMed]
- American Society for Metabolic and Bariatric Surgery. Story of obesity surgery 2018. [updated 2018, access January 20, 2019]. Available online: https://asmbs.org/resources/story-of-obesity-surgery
- American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. [Access January 10, 2019]. Available online: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers
- Angrisani L, Santonicola A, Iovino P, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg 2018;28:3783-94. [Crossref] [PubMed]
- Ugale S, Vennapusa A, Katakwar A, et al. Laparoscopic bariatric surgery-current trends and controversies. Ann Laparosc Endosc Surg 2017;2:154. [Crossref]
- Valli PV, Gubler C. Review article including treatment algorithm: endoscopic treatment of luminal complications after bariatric surgery. Clin Obes 2017;7:115-22. [Crossref] [PubMed]
- Monaco-Ferreira DV, Leandro-Merhi VA. Weight regain 10 years after Roux-en-Y gastric bypass. Obes Surg 2017;27:1137-44. [Crossref] [PubMed]
- Juza R, Rogers A. PEG and PEJ in the post-bariatric surgery patient. In: Pauli E, Marks J, eds. Percutaneous Endoscopic Gastrostomy (PEG): Techniques, Effectiveness and Potential Complications: Hauppauge, NY: Nova Science Publishers, 2016.
- Attam R, Leslie D, Freeman M, et al. EUS-assisted, fluoroscopically guided gastrostomy tube placement in patients with Roux-en-Y gastric bypass: a novel technique for access to the gastric remnant. Gastrointest Endosc 2011;74:677-82. [Crossref] [PubMed]
- Shaikh SH, Stenz JJ, McVinnie DW, et al. Percutaneous gastric remnant gastrostomy following Roux-en-Y gastric bypass surgery: a single tertiary center's 13-year experience. Abdom Radiol (NY) 2018;43:1464-71. [Crossref] [PubMed]
- Buwen JP, Kammerer MR, Beekley AC, et al. Laparoscopic sleeve gastrectomy: the rightful gold standard weight loss surgery procedure. Surg Obes Relat Dis 2015;11:1383-5. [Crossref] [PubMed]
- O’Brien PE, McPhail T, Chaston TB, et al. Systematic review of medium-term weight loss after bariatric operations. Obes Surg 2006;16:1032-40. [Crossref] [PubMed]
- McShea S. Obesity. Physician Assistant Clin 2017;2:87-106. [Crossref]
- Marchesini JCD, Noda RW, Haida VM, et al. Transenteric ERCP for treatment of choledocholithiasis after duodenal switch. Surg Laparosc Endosc Percutan Tech 2017;27:e28-e30. [Crossref] [PubMed]
- Schouten R, van Dielen FM, van Gemert WG, et al. Conversion of vertical banded gastroplasty to Roux-en-Y gastric bypass results in restoration of the positive effect on weight loss and co-morbidities: evaluation of 101 patients. Obes Surg 2007;17:622-30. [Crossref] [PubMed]
- Milone M, Elmore U, Musella M, et al. Safety and efficacy of laparoscopic wedge gastrectomy for large gastrointestinal stromal tumors. Eur J Surg Oncol 2017;43:796-800. [Crossref] [PubMed]
- Nunobe S, Hiki N. Function-preserving surgery for gastric cancer: current status and future perspectives. Transl Gastroenterol Hepatol 2017;2:77. [Crossref] [PubMed]
- Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Contin Educ Anaesth Crit Care Pain 2014;14:171-5. [Crossref]
- Blouhos K, Boulas KA, Tsalis K, Hatzigeorgiadis A. Management of afferent loop obstruction: Reoperation or endoscopic and percutaneous interventions? World J Gastrointest Surg 2015;7:190-5. [Crossref] [PubMed]
- Cui LH, Son SY, Shin HJ, et al. Billroth II with Braun enteroenterostomy is a good alternative reconstruction to Roux-en-Y gastrojejunostomy in laparoscopic distal gastrectomy. Gastroenterol Res Pract 2017;2017:1803851 [Crossref] [PubMed]
- Paye F. The pancreatic stump after pancreatoduodenectomy: the "Achille's heel" revisited. J Visc Surg 2010;147:e13-20. [Crossref] [PubMed]
- Donahue TR, Reber HA. Surgical management of pancreatic cancer--pancreaticoduodenectomy. Semin Oncol 2015;42:98-109. [Crossref] [PubMed]
- Shao XD, Qi XS, Guo XZ. Endoscopic retrograde cholangiopancreatography with double balloon enteroscope in patients with altered gastrointestinal anatomy: a meta-analysis. Saudi J Gastroenterol 2017;23:150-60. [PubMed]
- Watanabe M, Mine S, Nishida K, et al. Reconstruction after esophagectomy for esophageal cancer patients with a history of gastrectomy. Gen Thorac Cardiovasc Surg 2016;64:457-63. [Crossref] [PubMed]
- Farnes I, Johnson E, Johannessen HO. Management of gastric conduit retention following hybrid and minimally invasive esophagectomy for esophageal cancer: two retrospective case series. Int J Surg Case Rep 2017;41:505-10. [Crossref] [PubMed]
- Nguyen NT, Rudersdorf PD, Smith BR, et al. Management of gastrointestinal leaks after minimally invasive esophagectomy: conventional treatments vs. endoscopic stenting. J Gastrointest Surg 2011;15:1952-60. [Crossref] [PubMed]
- Frazzoni M, Piccoli M, Conigliaro R, et al. Laparoscopic fundoplication for gastroesophageal reflux disease. World J Gastroenterol 2014;20:14272-9. [Crossref] [PubMed]
- Smith CD, McClusky DA, Rajad MA, et al. When fundoplication fails: redo? Ann Surg 2005;241:861-9; discussion 869-71. [Crossref] [PubMed]
- Mosby's Medical Dictionary. anastomosis. (n.d.) 2009 [updated 2009, 8th ed] Available online: https://medical-dictionary.thefreedictionary.com/anastomosis
- Arezzo A, Bini R, Lo Secco G, et al. The role of stents in the management of colorectal complications: a systematic review. Surg Endosc 2017;31:2720-30. [Crossref] [PubMed]
- Abbas MA. Endoscopic management of acute colorectal anastomotic complications with temporary stent. JSLS 2009;13:420-4. [PubMed]
Cite this article as: Morrell D, Pauli E, Juza R. Endoscopy in surgically altered anatomy. Ann Laparosc Endosc Surg 2019;4:41.


