
History of right colectomy for cancer
Introduction
Putting right colectomy into historical context requires an understanding of the history of surgery in general. The first accounts of bowel surgery were often in response to trauma, specifically that of penetrating injuries. One of the earliest descriptions of treating an intestinal wound was attributed to Sushruta, a prolific contributor to medicine in East India in the 6th century BC. He stated that if the intestine protruded from the wound, it was to be “examined carefully, washed with milk, and gently replaced in its natural position”. If the intestine was perforated, he recommended the application of black ants to close the rent before replacing it back into the abdomen. Hippocrates, in the 4th century BC, considered intestinal wounds to be ‘invariably fatal’ and this was the western opinion held for several centuries thereafter. In the words of Ruggero de Frugardo: “If a part of the tender intestine is wounded, it is better to leave the treatment to God than to man, since death will follow it very soon.” (1)
Surgery, briefly
As civilization advanced, so did medicine and surgical treatment. In the 8th century AD, Rhazes used sheep gut to suture wounds instead of relying on black ants (1). Others used silk or linen suture and wrapped the sutured intestine in the intestine of an animal. Barber-surgeons performing blood-letting and boil-lancing have been well-known since the 14th century. Early surgeons developed inventive ways of aiding nature to heal injured bowel, such as suturing a dog’s trachea into the lining of the gut, as performed by Duverger of France in 1757 (1). The advent of anesthesia in 1843 allowed for larger, more complex operations while the patient was in an ‘insensate’ state (1,2). Listerism, Joseph Lister’s antiseptic principles which involved washing hands and instruments with carbolic acid, was responsible for reducing surgical morbidity to less than 50% after his paper was published in 1867 (3,4). And yet, abdominal surgery was avoided since entering the abdomen was considered to be noli me tangere—do not touch—(5) and usually fatal. Therefore, colon surgery was relegated to rare case reports. Interestingly, there is quite an extensive discussion on the history of ostomy surgery. The first ostomies were accidental in that they were actually enterocutaneous fistulas as a consequence of perforated bowel (6).
Entering the abdomen
In the pre-Listerian era, an individual with intra-abdominal pathology such as perforated viscus was often left untreated. Upon recognizing an intra-abdominal abscess, the surgeon would close the abdomen and let the disease take its course. Post-Lister, emboldened with the help of anesthetics and antisepsis, Bobbs performed the first gallbladder operation in 1868 (7). In 1886, Fitz performed the first successful appendectomy for appendicitis (8).
Colon surgery, or, colostomies
The first foray into colon surgery was because of bowel obstruction, often due to an advanced tumor. Paracelsus, in the 15th century, was thought to be the first to propose the opening of intestine to the abdominal wall to relieve obstruction (1). In 1776, Pillore of Rouen suggested the creation of an “artificial anus”, or ostomy, diverting the intestinal tract away from the obstruction caused by a large rectal tumor. Other doctors at the time opposed the operation, but the patient insisted it be done. Pillore made a transverse incision above the groin and “drew up the cecum and opened it transversely and stitched it to the two lips of the abdominal wound”. The man died 20 days later, but other successful colostomy procedures followed, including that described by Amussat, which is now known as Amussat’s procedure (1).
Colon resections
In 1823, Reybard is credited with performing the first sigmoid colon resection with an end-to-end anastomosis. The patient survived but at the time such an operation was discouraged as being drastic and dangerous. Reybard was famously criticized by the Paris Academy of Medicine for performing this operation. Billroth resected a portion of colon and brought the proximal end out as a colostomy (1,9). Thiersch was also known to have performed a colon resection for acute obstruction. By 1880, only 10 large bowel resections had been recorded and only 3 were considered successful. Weir, in 1883, published the results of 33 cases of intestine resection with ‘artificial anus’ with 51% mortality (10). Gussenbauer, Czerny, Bryant and Heineke attempted operations where the obstructing lesion was exteriorized, the abdomen closed, and the mass and colon resected several days later in a two-stage operation (1).
With the advent of the concept of exteriorization, colon resections met greater success with less morbidity. Resection with proximal decompression and delayed anastomosis was reported by Bloch in Copenhagen in 1890 but was famously described in 1902 by Mikulicz in the operation that bears his name. He wrote: “If now the tumor has been freed and completely enucleated, it is drawn out of the wound, the loop of gut is stitched to the parietal peritoneum with sutures including only the serous coat, and the abdominal wound is closed, leaving only room enough for the loop of gut. Now, only after the abdominal cavity is completely closed, the tumor is excised, and an artificial anus is established which is closed in two to four weeks, according to the usual methods (1).” Henry Hartmann was also a crucial figure in this history, popularizing the concept of resection with diversion and closing the rectum for rectal cancer (6). At the turn of the century, Sir William Arbuthnot Lane published his observations on 39 colectomies that he performed at Guy’s Hospital in London. He performed these total colectomies for chronic constipation or what he called ‘autointoxication’ (11). Though his contributions were later ridiculed, he is considered to be one of the pioneers of colon surgery for routinely resecting the offending colon and performing an ileorectal or ileosigmoid anastomosis, known as Lane’s operation.
On anastomoses
Various methods were used to join the ends of intestine in an anastomosis—“reed pipe, goose trachea, cardboard smeared with sweet oil, a cylinder of fish glue, a wax ring, and a silver ring, to name a few” (5). Murphy’s button was introduced in 1892 and used widely as a sutureless anastomosis (5). Three years later, Abbe resected a portion of cecum and used a Murphy button to create an enterocolostomy. The patient died from obstruction of the button lumen with feces. By the 1900’s the button had fallen out of use, with most surgeons preferring absorbable vegetable plates or suture alone (1) (Figure 1).
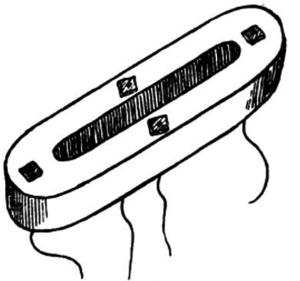
Right colectomy
One other method of adjoining bowel was mentioned in Lowson’s delightful paper in The Lancet, published March 25, 1893, the earliest mention of a right colectomy with a primary anastomosis. A 33-year old man who “took part in a pugilistic encounter with a pugnacious friend” at a local fair “emerged from the dust and din of battle, victorious” but was later attacked by “friends of the vanquished”. They left him bruised and beaten, half dead for several hours until he crawled home at 4:00 am. He suffered right-sided abdominal pain for months but didn’t seek any medical attention until 6 years later when he had a “severe haemorrhage from the bowels, when quite a pint was lost”, and then again, a month later, “nearly as profuse”. At this point, he noticed a swelling in right abdomen and was seen in consultation by a surgeon who found “a tumor the size of a cocoanut [sic] situated in the right loin”. The mass was firm, movable and tender, but did not cause any obstruction. What follows is a description of the operation:
“…I opened the abdomen…by a vertical incision in Langenbuch’s position to the outer side of the rectus. A large mass immediately presented itself, involving the middle of the ascending colon, the caecum and appendix being unaffected. The omentum was adherent to the tumor anteriorly, and this I severed with scissors and ligature. Pushing the colon inwards, I now entered the scissors above the level of the tumour through the posterior layer of peritoneum lining the posterior wall of the abdomen to the outer side of the great bowel, and ran it down to a point opposite the lower end of the caecum. The bowel could now be easily separated from its bed. It still remained to divide the peritoneum on the inner side where the colic vessels spread out fan-like to supply the colon. This was done by tying the serous membrane with the vessels in five or six successive pieces and dividing distally –i.e., between the ligatures and colon. The line of this incision inclined downward and inward, meeting the ileum as it crossed to join the colon five or six inches from the ileo-caecal valve. Several diseased glands were included in this triangle. The ileum was separated from the mesentery in the same way, and now the greater part of the ascending colon, with the caecum and four or five inches of the ileum, were free along with the tumor.”
The bowel was then divided with clamps and oversewing the stumps, first with a silk continuous suture and a second layer with Lembert’s sutures. The anastomosis reads as such:
“I…opened the transverse colon anteriorly, the opening being rather more than an inch long and running in the long axis of the bowel. Into this one of Senn’s bone-plates was introduced with the threads in position—i.e., two placed laterally and one at each end. The lateral threads were drawn through the intestinal wall and the end ones made to come out at the angles of the wound. The ileum was treated in the same way and a second bone-plate similarly introduced.”
The sutures were tied together, the bowel was washed and the sponges were removed before closing the abdomen. He kept a Keith’s tube in for several days. The patient tolerated hot milk on the third day, and was discharged home after 25 days in the hospital. Apparently on follow-up he had gained weight and won a foot race. Of note, the author added that he found several points that were unnecessary in using the bone-plates, and one that was essential:
“One is, after the introduction of the plates and before tying the ligatures, to scratch the serous surfaces to be apposed; the second is to anchor the bowel in the vicinity of the junction to the parietal peritoneum; and the third to attach round the place of union an omental graft. One detail…cannot be neglected without fatal extravasation of intestinal contents is to be especially particular to bring the serous surfaces accurately in apposition at the point where the mesentery joins the intestine and where the serous coat of the colon is deficient behind.”
Thus, D. Lowson of Aberdeen University was a pioneer of the open right colectomy, describing salient points that are recognizable today, such as dissection of the white line of Toldt and the division of the mesentery. Some techniques, such as bone-plates for anastomosis, are less familiar. Senn designed the bone-plates as a method of stenting and sealing the anastomosis (Figure 2). The operation was successful and the patient lived and, in fact, “thrived post-operatively” (12).
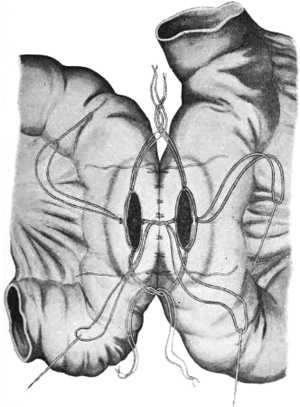
Starr, in 1922, published “The Surgery of the Right Bowel” in Surgery, Gynecology and Obstetrics. He noted that post-appendectomy patients may develop recurring pain and nausea which “passes for chronic appendicitis” but he describes what sounds like a cecal bascule: “to clinch the diagnosis, an opaque enema is given and with the X-ray one sees the caecum balloon and remain ballooned, then the barium begins to flow through the ileocaecal valve”. He performed twenty ileocolic resections in 1920 and eighteen in 1921. There were five deaths due to “colon infections in the wound”. In his later series he utilized a piece of rubber tubing, stenting the anastomosis. The tube was passed from the rectum in 5 to 15 days (13).
Muir wrote in 1943 of a procedure that utilized a Millar-Abbott tube stitched into the ileostomy end of a side-to-side antiperistaltic anastomosis for temporary diversion. The tube was removed in a week and the fistula closed in ‘a fortnight’, making it a one-stage right hemicolectomy (14) (Figure 3). It is worth noting that he names Paul as having successfully completed a right colon resection in 1895, and Gabriel as devising the method of catheter drainage at the anastomosis. In 1945, Shier referenced Starr and employed a rubber tube stent in his right colectomy. Shier wrote of an additional step of dusting his anastomosis with sulfanilamide (15).
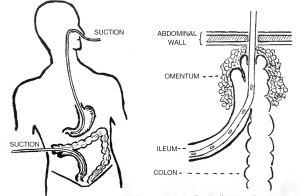
At the 9th Christian Medical Association in Vellore, India in 1944, Arawattigi presented his paper on ‘Resection of the right half of the colon (hemicolectomy)’. He wrote, “In the beginning, colostomy as a decompressive measure was the only operation performed for cancer, but gradually enterprising and persistent surgeons doggedly extended the horizon and began to attempt the removal of malignant lesions and the establishment of gastro-intestinal continuity.” Arawattigi writes that for cancer, ‘total extirpation’ is “‘probably’ the procedure of choice, but it is associated with ‘too high a mortality to be of practical value’” (16).
Arawattigi presents a series of 40 cases in which he performs a one-stage right colectomy with primary anastomosis, even though he stated that two-stage operations were preferred, having a 20% mortality versus 32% in a one-stage operation. Arawattigi utilized a right paramedian incision and pulled up the hepatic flexure with a gauze passed through the mesentery. The entire colon is mobilized. Then “a rubber-covered anastomosis clamp is placed over the ileum proximal to the level of resection, and a crushing clamp at the distal level, and the gut is divided by an electric knife.” An end-to-end anastomosis was made with catgut suture. The patients were able to take regular food on the “9th or 10th post-operative day”, though liquids were given on the first post-operative day. In his series, there were two mortalities with the single-stage operation and no mortalities with the two-stage operation, but he advocates for the single-stage operation largely due to the fact that dense adhesions were encountered on opening the abdomen for the second stage, writing “there is no advantage in opening the abdomen twice” (16).
Corman’s text describes the contemporary open right hemicolectomy as follows:
“Lesions of the cecum, ascending colon and hepatic flexure are treated by right hemicolectomy because the blood supply to this area comes from the ileocolic and right colic arteries. The dissection may be expedited if the surgeon stands on the patient’s left side, although this is a matter of personal preference… The small bowel is retracted into the left half of the abdominal cavity, and the root of the mesentery and the base of the transverse mesocolon are exposed. Many surgeons ligate the right colic and right branch of the middle colic arteries and veins as the initial maneuver… The small incision in the root of the mesentery…is now extended to the point on the transverse colon and ileum where division of the bowel is to take place. The vessels in the mesentery and mesocolon are ligated, and the entire blood supply to the tumor is divided (Figure 4). An alternative to ligating the mesenteric blood supply is to employ a vessel-sealing device, such as the LigaSure vessel sealing system…The terminal ileum and right colon are elevated from the retroperitoneal structures by dividing the peritoneum along the lateral gutter, at the so-called white line of Toldt… The terminal ileum is prepared by incising the antimesenteric fold of Treves…the duodenum…must be displaced carefully as the colon is freed from it’s retroperitoneal attachments. The developmental adhesions from the gallbladder and liver to the hepatic flexure are incised. When the head of the pancreas is in view, the duodenum is sufficiently clear from the dissection so that clamping of the blood supply can be accomplished with safety (Figure 5)…It is often advisable to enter the lesser sac, dividing the gastrocolic omentum as far to the left as possible…this maneuver…permits the posterior wall of the stomach to be retracted out of harm’s way. The remainder of the gastrocolic omentum is then divided until the hepatic flexure is no longer tethered…With all the blood supply divided to the segment, the bowel is resected.”
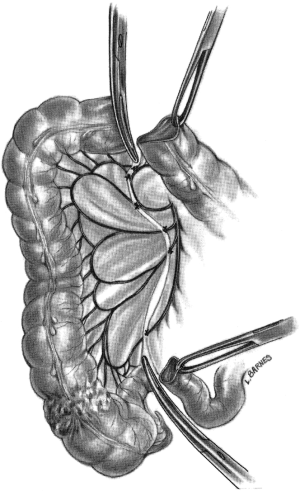

If performing a hand-sewn anastomosis, a Cheatle cut into the antimesenteric portion of the ileum may be useful to accommodate the size discrepancy between the ileum and the transverse colon (Figure 6). Suturing of the end-to-end anastomosis is done with a single layer, interrupted, inverting technique with adequate bites of the submucosal strength layer and small bites of the mucosa. Though a second seromuscular layer of stitches is preferred by some surgeons, it is not necessary and may narrow the anastomosis. The rent in the mesentery may then be closed, especially if it is a small opening, and it is prudent to place the omentum around the anastomosis (5).
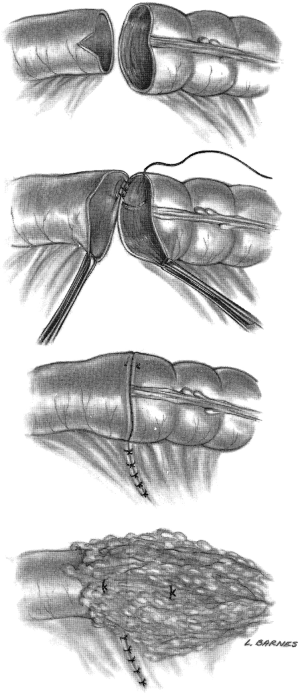
Henroz first tested a stapling device on dogs in the 1800s. This device allowed for the approximation of everted tissues. Several iterations of the gastrointestinal (GIA) stapler followed, including the precursors of the modern stapler, which were developed post World War II in the former USSR. Since the popularization of the GIA stapler in the 1970’s, the ileocolic anastomosis is often performed in a functional side-to-side fashion with a stapling device. The anastomosis may also be performed in a side-to-side fashion or end-to-end with a circular stapler (17).
The pearls in each iteration of right colectomy are evident, and the lessons through its history are many. The principles of mobilization of the colon, careful dissection avoiding important surrounding structures, and primary anastomosis have been well-established. As is the case with any operation, the current status of right colectomy relies on the innovation of those who came before us. While the incidence and morbidity have been greatly reduced, newer approaches are needed to further improve results. There is still a long way to go, but it is wise to reflect on how far we have come.
Acknowledgments
Jessica Koos, MLS, MSEd, AHIP at Stony Brook Health Sciences Library and Arlene Shaner, MA MLS at New York Academy of Medicine.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Bergamaschi and Mahir Gachabayov) for the series “Right Colon Cancer Surgery: Current State” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.05.05). The series “Right Colon Cancer Surgery: Current State” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meade RH. An Introduction to the History of General Surgery. Philadelphia: W.B. Saunders, 1968:261-90.
- Bigelow HJ. Insensibility during surgical operations produced by inhalation. Boston Med Surg J 1846;35:309-17. [Crossref]
- Lister J. On a new method of treating compound fracture, abscess, etc., with observations on the conditions of suppuration. Lancet 1867;89:387-9. [Crossref]
- Gawande A. Two hundred years of surgery. N Engl J Med 2012;366:1716-23. [Crossref] [PubMed]
- Corman ML. Colon and Rectal Surgery. Sixth edition. Philadelphia: Lippincott Williams & Wilkins, 2012.
- Doughty DB. History of ostomy surgery. J Wound Ostomy Continence Nurs 2008;35:34-8. [Crossref] [PubMed]
- Bobbs JS. Case of lithotomy of the gallbladder. Trans Indiana State Med Soc 1868;18:68-73.
- Williams GR. Presidential Address: a history of appendicitis. With anecdotes illustrating its importance. Ann Surg 1983;197:495-506. [Crossref] [PubMed]
- Wangensteen OH, Wangensteen SD. The Rise of Surgery. Minneapolis, Minnesota: University of Minnesota Press, 1978.
- Weir RF. II. Resection of the Large Intestine for Carcinoma. Ann Surg 1886;3:469-89. [Crossref] [PubMed]
- Lane WA. Remarks on the results of the operative treatment of chronic constipation. Br Med J 1908;1:126-30. [Crossref] [PubMed]
- Lowson D, Aberd CM. Resection of the caecum, greater part of ascending colon and five inches of ileum for malignant disease of ascending colon. Lancet 1893;141:648-9. [Crossref]
- Martin FH, Kanavel AB. The surgery of the right bowel. Surg Obstet Gynecol 1922;34:47-8.
- Muir, EG. Right hemicolectomy. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2184220/pdf/procrsmed00528-0073.pdf
- Shier RV. Right Colon Resection. Can Med Assoc J 1945;53:18-20. [PubMed]
- Arawattigi SD. Resection of the Right Half of the Colon (Hemicolectomy). Ind Med Gaz 1944;79:7-10. [PubMed]
- Baker RS, Foote J, Kemmeter P, et al. The science of stapling and leaks. Obes Surg 2004;14:1290-8. [Crossref] [PubMed]
Cite this article as: Fong CF, Corman ML. History of right colectomy for cancer. Ann Laparosc Endosc Surg 2019;4:49.


