Endoscopic enteral access
Introduction
Being able to maintain appropriate nutrition in ill patients is a fundamental part of caring for both surgical and medical patients. The literature supports that malnutrition is associated with adverse clinical outcomes. Therefore, every effort should be made to support nutritional status when a patient is acutely or chronically ill. A study by Naber et al. found that the severity of malnutrition in patients can predict the occurrence of complications during their hospital admission (1). Tappenden et al. found evidence to suggest that early nutritional support can reduce complication rates, length of hospital stay, readmissions, mortality, and costs of care (2).
In patients who have an accessible and functional gastrointestinal system, enteral nutrition is the preferred route. For patients who require permanent or long-term (greater than four weeks) access to nutrition, enteral nutrition through an enterostomy tube is a good option that can be done by endoscopy, radiologically or surgically (3). Enteral nutrition reduces the risk of morbidity and cost when compared to parental nutrition (4-6). Compared to parenteral nutrition, enteral nutrition via a feeding tube has been associated with a decreased risk of infection (6). Also, in a systematic review, authors found evidence to suggest that there is an economic advantage of enteral nutrition over parental nutrition (4). In patients that require enteral nutrition for less than four weeks, nasoenteric tubes should be considered. However, a study comparing percutaneous endoscopic gastrostomy tubes (PEG) versus nasogastric tubes (NGT) found that PEG was associated with a lower risk of intervention failure, was more effective, and safe when compared to NGT (7).
Endoscopic techniques have been associated with faster recovery, lower costs, and can be less invasive when compared to open surgical procedures (4). Grant et al. identified that the overall complication rate after PEG was lower when compared to those undergoing open gastrostomies (8). Furthermore, PEG was also associated with shorter operative time, no need for general anesthesia, lower cost, a lower incidence of complications, and required less recovery time when compared to open surgery (8,9). Al-Abboodi et al. found that there was no difference in bleeding, surgical site infection or mortality in patients undergoing PEG placement who have cirrhosis (10). Endoscopic enteral access is a safe and practical procedure for patients who require nutritional support. This article reviews the indications, contraindications, pre- and post-procedure care, and procedure techniques for endoscopic enteral access.
Periprocedural assessment
Indications
Before placing enteral access, patients should be unable to maintain adequate nutrition via oral feeds alone. Typically, nutritional support should be considered if the recommended daily dietary requirements will not be met for more than seven days in an adult. However, early nutritional interventions may be indicated if the patient is malnourished. Additionally, patients must have a functional gastrointestinal system and able to tolerate intraluminal feeding.
When starting enteral feeds, one should consider the best route of providing nutritional support. Most patients can tolerate intragastric feeding although there are times when other routes should be entertained. When considering intragastric feeding, the lower esophageal sphincter should function properly to avoid gastric reflux into the esophagus. Furthermore, the stomach should be able to work as a reservoir and propulsive organ. Jejunal feeding should be considered if the patient has recurrent aspiration of gastric content, delayed gastric emptying or esophageal dysmotility with regurgitation (3,4). The disadvantage of jejunal tubes, particularly ones that are placed via a gastric extension, are the technical difficulty of placing and maintaining the tube post-pyloric. There may also be an element of feeding intolerance when feeds are directly entered into the jejunum. Gastric feeding has the advantage of being more convenient (can be given as a bolus), straightforward (does not require a pump) and physiologic. Intragastric feeds can buffer gastric acid and help regulate gastric emptying by humoral and neural pathways better than when receiving jejunal feeds (11,12).
Common indications for enteral tube access for gastric or jejunal feeding includes physiologic anorexia, neuromuscular swallowing disorders, gastrointestinal (GI) malabsorption, decreased consciousness, and upper GI tract obstruction. Other indications include injuries that increase the catabolic state of a patient such as severe burns or illness, malignant or benign tumors, cystic fibrosis, mental health issues, and intraabdominal fistulas (Table 1) (4).
Table 1
| Indications | Types of diseases for each indication |
|---|---|
| Gastrointestinal | Tumor |
| Esophageal stricture (benign) | |
| Short bowel syndrome | |
| Enterocutaneous fistula | |
| Gastric decompression | |
| Reduction of volvulus | |
| Neurologic | Brain tumor |
| Cerebrovascular accident | |
| Motor neuron disease (multiple sclerosis or Parkinson’s disease) | |
| Mental health (severe depression or anorexia nervosa) | |
| Dementia or Alzheimer’s | |
| Traumatic brain injury | |
| Critical illness or physiologic anorexia | Malignancy |
| Sepsis or multiorgan dysfunction | |
| Hepatic disease | |
| Cystic fibrosis | |
| Severe burns | |
| Immunodeficiency (HIV/AIDS) | |
| Prolonged ventilation or coma | |
| Intensive care patients | |
| Miscellaneous | Face, head, or neck surgery |
| Face or oropharyngeal trauma | |
| Chronic renal failure | |
| Jejunal feeding | Gastroparesis |
| Diffused or infiltrative gastric cancer | |
| Severe GERD | |
| Gastric outlet obstruction | |
| Severe acute pancreatitis | |
| Variations in gastric anatomy (gastric bypass or post gastrectomy) | |
| Gastric or duodenal fistula |
HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; GERD, gastroesophageal reflux disease.
Patients who have a previous past medical history of delayed gastric emptying, severe reflux or esophagitis, or pulmonary aspiration, jejunal enteral feeds should be considered (3,4). The advantages of jejunal enteral feeding include minimizing the risk of aspiration, provide nutrition for patients with gastric outlet obstruction, pancreatitis, infiltrative gastric cancer, variations of gastric anatomy (gastric bypass or post gastrectomy), gastroparesis, severe gastroesophageal reflux disease (GERD), or intraabdominal fistulas (4).
Contraindications
Patients with oropharyngeal or esophageal obstruction or multiple facial injuries are a relative contraindication for oral and enteral nutrition. Therefore, percutaneous enterotomy should be considered (3,4). Other relative contraindications include abdominal wall hernias, extreme obesity, previous upper GI surgery, ascites, peritoneal dialysis (PD), carcinomatosis peritonei, gastric ulcers, ventriculoperitoneal (VP) shunts, pregnancy, portal hypertension with gastric varices, the presence of a stoma, or surgical scars that interfere with enteric tube placement. Absolute contraindications for enteral access include having no informed consent, hemodynamic instability, and uncorrectable coagulopathy (Table 2) (3,4).
Table 2
| Absolute | Relative |
|---|---|
| Mechanical obstruction of the GI tract* | Obstruction of the oropharynx or esophagus |
| Uncorrectable coagulopathy | Peritoneal Carcinomatosis |
| Peritonitis | VP-shunt or peritoneal dialysis |
| Ischemic bowel | Pregnancy |
| Hemodynamic instability | Gastric ulcer |
| Gastric varices or ascites secondary to portal hypertension | |
| Unstable facial fractures | |
| Extremely obese |
*, Unless the procedure is indicated for decompression. VP, ventriculoperitoneal.
Preprocedure evaluation
Informed consent should be obtained from the patient or surrogate prior to the procedure. Patients should be advised to not eat solid food or liquids, six and three hours, respectively, before their procedure. The risks and benefits of stopping anticoagulants or antiplatelet medications should be weighed to the risk of bleeding before the procedure. The use of these medications might increase the risk of bleeding during and after the endoscopic procedure. The patient’s unique characteristics and preexisting comorbidities should be considered before discontinuing antithrombotic medications for a short period, taking into consideration the patients risk of having a thromboembolic event (deep venous thromboembolism, pulmonary embolism, and cerebral vascular accident) (13).
Antibiotic prophylaxis should be given 30 minutes before percutaneous endoscopic enterostomy (PEE) to reduce the risk of peristomal infection (14). Antiseptic skin agents should also be used to reduce the risk of infection. A systematic review found that patients who received prophylactic antibiotics before PEG tube placement had decreased the odds of developing an infection compared to those who did not receive antibiotics (15).
Endoscopic enteral tube access techniques
Endoscopy guided nasoenteric tube placement
The technical success rate of endoscopy guided nasoenteric tube (ENET) placement is higher than 90% (3). The ENET can be placed at the bedside with or without sedation. There are several placement methods which can be used. The most common include the pull and drag method, which has been used the longest. First, a suture is placed at the distal end of a feeding tube and inserted through the nose down into the stomach. The suture is then grasped using either a forcep or hemostatic clip and dragged from the stomach into the jejunum (3,4). To help avoid migration of the enteral tube, when the endoscope is removed, a hemostatic clip can be used to secure the suture to the mucosa of the jejunum (4). ENETs can also be placed using an over-the-wire technique. First, a wire is placed in the biopsy channel of the endoscope and placed into the stomach or jejunum. Next, the scope is removed while maintaining the wire in place. Finally, the enteral tube is passed over the wire and directed into the stomach or jejunum and then repositioned at the back end to exit the mouth (nasal transfer) (3,4). Fluoroscopy is often used to aide and confirm the placement of the tube. Another approach is to use an ultrathin endoscope that can be passed directly through the nose into the stomach. A guide wire can then be advanced into the jejunum through the endoscope. Next, you remove the endoscope while the guide wire is “exchanged” for the scope or left in place. The tube can then be passed over the wire into the jejunum (3,4). Next, a therapeutic endoscope is guided into the small bowel, and a feeding tube (8- or 10-F) is advanced through the therapeutic channel (3.7-mm). The endoscope is removed while maintaining the end of the tube in position. The last steps are to perform a nasal transfer and attach the feeding adapter to the end of the enteral tube (3,4).
Percutaneous endoscopic gastrostomy
The technical success rate of PEG tube placement ranges between 76% and 100% (16). The benefit of using the endoscopic approach (over a surgical or radiologic approach) is the ability to do it at the bedside. Common causes for the unsuccessful placement of a PEG tube is inadequate transillumination, complete obstruction of the oropharynx or esophagus, and previous gastric surgery (16). Studies have found that PEGs can be safely passed through oropharyngeal or esophageal obstructions using an ultrathin endoscope (17-19). PEG tubes can be placed either trans-orally or trans-abdominally. Studies which have compared techniques for placing PEG tubes have found comparable success rates and length of time of the procedure (4,20,21).
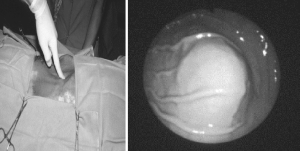
The first technique we will be discussing is the trans-oral route. First, the stomach is insufflated with air or carbon dioxide using the endoscope. The gastric and abdominal wall is then indented with a finger while visualizing the indention endoscopically (Figure 1). The abdomen is prepared and draped in a sterile fashion, and local anesthesia is injected into the abdominal wall and peritoneum. Next, a needle on a syringe filled with saline is inserted into the abdominal cavity while simultaneous aspirating using the “safe tract” method (4). Afterward, a scalpel is used to make an incision at the previously designated site, followed by placing an introducer needle under direct visualization using endoscopy. A guide wire is then passed through the introducer needle into the abdominal cavity, and a snare or forceps is used to grasp the guide wire via the endoscopes working channel. The wire is then removed along with the endoscope. With the remaining wire that is exiting the mouth, a feeding tube is attached to the external end and pulled back together from the mouth into the stomach and through the abdominal wall. The final step is to place an external bumper approximately one centimeter above the abdominal skin to allow movement of the tube into the stoma to help avoid pressure necrosis. A repeat endoscopy may be completed to check for hemostasis and determine the best location of the tube (3,4).
In the transabdominal approach, the initial steps are similar to the previous technique. A needle on a syringe is inserted through the abdominal wall into the gastric cavity under direct endoscopic visualization, and a guide wire is introduced through the needle. However, in this technique, the track is dilated over the wire to allow for direct placement of the tube under endoscopic visualization. Next, using a peel-away sheath, a balloon-tip gastrostomy catheter is positioned into the stomach (4). Gastric insufflation is usually lost during the introducing phase. Therefore, gastropexy with T-fasteners or a gastropexy device can be helpful (3,4). The primary benefit of this technique is the avoidance of pushing or pulling the endoscope through the oral cavity. Also, you avoid tumor seeding of head and neck cancers at the stoma site, and there may be an infectious benefit.
Percutaneous endoscopic gastrostomy with jejunal extension
The success rate of percutaneous endoscopic gastrostomy with jejunal extension (PEGJ) is around 90–100% (3). An endoscope is passed trans-orally into the stomach, and a guide wire is inserted into a previously placed gastrostomy tube. The tube is usually of greater diameter (26 French), to allow the jejunal tube to fit through the gastrostomy tube. The wire is grasped with forceps and directed into the proximal small bowel. A jejunal tube is then advanced over the guide wire to the desired position, followed by removing the wire and forceps. Next, the jejunal extension is fitted into the gastrostomy tube. A similar technique that can be used is to pass a guide wire into a mature gastrostomy tract into the proximal tract using the previous method described. This would allow the use of one device, instead of two, which incorporates both the gastrostomy and jejunal components into one tube. Also, a 5–6 mm diameter ultrathin endoscope can be introduced through the mature gastrostomy or gastrostomy tube and placed in the jejunum. The final step involves removing the endoscope and passing the jejunal tube over the guide wire (3,4).
Direct percutaneous endoscopic jejunostomy
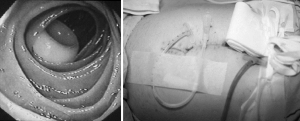
The technical success rate of direct percutaneous endoscopic jejunostomy (DPEJ) has been reported between 68% and 100% (4). Poor transillumination is the most common cause of failure when placing a DPEJ (3). Poor transillumination can be caused by increased thickness of the abdominal wall or omentum, therefore, the procedure has higher success rates in thin patients (22). Also, in native anatomy, getting beyond the ligament of Treitz can be challenging using a standard gastroscope. Some authors have suggested the use of a balloon-assisted overtube, fluoroscopy and leaving the overtube in place during the entire procedure with a reported success rate of 96% (23). Other techniques and maneuvers include using a stiff scope, such as, a pediatric colonoscope and using a stiff guidewire (24). Also, DPEJ can be more technically demanding than PEGJ, however, it is more durable and can have decreased need for re-intervention (3). It is imperative to have an accurate understanding of the patient’s anatomy when undergoing this procedure. Patients who require DPEJ often have altered anatomy (i.e., bypass or esophagojejunostomy). The stomach is insufflated with air. Next, the endoscope is passed into the jejunum and transilluminated. Next, under direct endoscopic visualization of the jejunum, the abdominal wall is indented with a finger while visualizing the indention. The abdomen is prepared and draped in a sterile fashion. Local anesthesia is injected into the abdominal wall and peritoneum. Next, insert a needle on a syringe filled with saline into the abdominal cavity while simultaneous aspirating using the “safe tract” method. An incision is made with a scalpel at the previously determined site. Next, under direct endoscopic visualization place an introducer needle into the jejunum. In patients who have had a prior Billroth II, the efferent limb should be identified by using fluoroscopy or identifying the Ampulla of Vater in the afferent limb by using the endoscope prior (22). Next, place a guide wire through the introducer needle and grasping it using snares or forceps via the working channel of the endoscope. The wire and endoscope are then removed together, and the remaining guide wire is attached to the outer end of the feeding tube. Next, the tube and guide wire are pulled back together from the mouth into the stomach and through the abdominal wall. A bumper is then placed externally about one centimeter above the skin of the abdomen to allow for movement of the tube into the stoma which can help circumvent pressure necrosis (Figure 2). A repeat endoscopy may be done to determine the best placement of the DPEJ tube and to evaluate for hemostasis (3,4). This technique often requires two skilled endoscopists, fluoroscopy and a thorough understanding of anatomy. The chosen location for direct jejunal tube placement is often close to the ligament of Treitz to avoid jejunal volvulus around the feeding tube. Patients with known adhesions are often easier to place this type of enteral tube as the small intestine will have less movement and allow for safer placement.
Difficult enteral access
Alimentary tract cancer
Patients with oropharyngeal or esophageal malignancy may develop malnutrition due to obstruction. The use of endoscopic guided tube placement can be used when the blind placement of nasogastric tube placement fails. Endoscopic guided nasoenteric tube placement can be considered for patients who will only require short term feeding. A 5–6 mm diameter endoscope is passed through the nose and tumor into the stomach with subsequent tube placement over a guide wire (3,4). Dilation of the esophageal obstruction is required at times to pass the endoscope through the obstruction. However, there is an increased risk of perforating the esophagus (3,4). A feeding tube nasal transfer may be needed if using the oral route. Patients who will require long term nutritional support or if an ENET cannot be performed may benefit from a PEG (3,4).
Pregnancy
PEG tube placement is a safe procedure without significant complications that be performed during pregnancy between 8 to 29 weeks of gestation (25). Although PEG tube placement is rare during pregnancy, it may be required when patients have severe hyperemesis gravidarum leading to inadequate oral intake and nutritional deficiencies which may lead to fetal morbidity or mortality (26). It is recommended to define the dome of the uterus using ultrasonography (25). To separate the PEG site from the rib cage and uterus, ultrasound indentation and transillumination may be used. Patients should undergo fetal monitoring throughout the procedure. As the uterus enlarges attention should be paid to the external bumper to ensure there is no pressure which may lead to pressure necrosis from the tension of the internal and external bumpers (25). Often, the loosening of the PEG is required as the fetus enlarges.
Ascites
Patients with ascites can pose a challenge when enteral access is required and was historically one of the few relative contraindications to PEG tube placement, but more recent literature has demonstrated it to be a safe procedure (4). Al-Abboodi et al. examined patients with liver cirrhosis and ascites that required PEG tube placement. The authors found that patients with ascites had no difference in bleeding, surgical site infections, urinary tract infections, and mortality when compared to patients without ascites (10). Ultrasound-guided paracentesis and gastropexy can be used to decrease peri catheter leakage and dislodgement (27). Often the aspiration of ascites is done before the procedure to decrease the risk of bacterial contamination of the fluid.
Peritoneal dialysis and ventriculoperitoneal patients
In patients who have VP shunts, PEG can also be placed safely; however, there is a risk of infection (28). PEGs should be placed as far away as possible from the VP shunt (29). Studies have found that patients who have PEGs placed after PD placement have a high rate of developing peritonitis (30,31). It is recommended that patients receive antibiotic and antifungal prophylaxis in addition to withholding peritoneal dialysis for two to three days or longer around the time of enteral tube placement (30,31).
Postprocedure care
The care of enteral tubes is similar to that of a nasogastric tube. Enteral feeds could be started right after ENET placement if there were no complications during the procedure. Typically, in patients who had a PEE placed, tube feeds are delayed 12–24 hours due to the concern of bleeding or intraabdominal leakage; however, studies have found that early feeding is safe and well tolerated (3,4). Every attempt should be made to irrigate the enteral tubes with water before and after each use to prevent clogging. If a tube becomes clogged, one should consider flushing with water, pancreatic enzymes, or bicarbonate solution. Other unclogging maneuvers include using a Fogarty balloon, biopsy brush, or commercial tube de-clogger (4). Tube replacement should be considered as the last resort.
Complications
Complications after enteral tube placement include pain at the site of tube insertion, pressure ulcer, esophageal perforation, reflux esophagitis, epistaxis, tube malposition, tube occlusion, tube dislodgement, leaking, bleeding, pneumoperitoneum, and diarrhea. Bleeding can occur up to 1% of the time, which is caused by injury to surrounding vessels and coagulopathy. Preventative measures can be taken such as identifying abdominal wall vessels using transillumination. Bleeding can be managed by temporarily tightening the external bumper, using endoscopy to identifying bleeding vessels or correcting any underlying coagulopathy (4). Bleeding can be managed endoscopically at the time of enteral tube placement, by using standard endoscopic methods such as hemostatic clips, energy and injection agents. Pneumoperitoneum can occur up to 56% of cases. The management will depend on the patient’s symptoms. In an asymptomatic patient, close observation is warranted. If the pneumoperitoneum persists over 72 hours or the patient develops worrisome symptoms, then a CT scan with water-soluble oral and enteral tube contrast can be used to evaluate for any contrast extravasation. If the patient develops peritonitis, then surgery would be indicated (4). The use of CO2 during enteral access (instead of room air) allows for faster absorption of the gas and may decrease the overall complications of pneumoperitoneum. In cases where there is migration of the jejunal tube into back into the duodenum tube, redirection is recommended. Lastly, peristomal granulomas can occur up to 27% of cases which can be prevented by proper wound care and can be managed by applying topical antimicrobials, low dose steroids, or silver nitrate (4). Many of these complications are relatively uncommon if the appropriate technique is maintained during placement and good tube and skin care are followed post placement (3,4) (Table 3).
Table 3
| Acute | Delayed |
|---|---|
| Pneumoperitoneum | Jejunal volvulus |
| Gastric perforation | Retrograde migration of the tube |
| Esophageal perforation | Gastroesophageal reflux or erosion |
| Bleeding | Peristomal granuloma or ulceration |
| Prolonged ileus | Gastric outlet obstruction |
| Aspiration | Enterocutaneous fistula |
| Tumor seeding | |
| Tube clogging or accidental removal | |
| Buried bumper syndrome | |
| Stoma leakage | |
| Necrotizing fasciitis | |
| Surgical site infection |
Conclusions
Endoscopic enteral access is a safe and practical procedure for patients who require nutritional support. There are various techniques with their relative safety profile and success rates as described. The technical approach should be individualized to each patient, taking into consideration patient anatomy, disease status, anatomic variances, and the practitioner’s skill level.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jeffrey M. Marks and Ryan M. Juz for the series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.05.04). The series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Naber TH, Schermer T, de Bree A, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr 1997;66:1232-9. [Crossref] [PubMed]
- Tappenden KA, Quatrara B, Parkhurst ML, et al. Critical role of nutrition in improving quality of care: an interdisciplinary call to action to address adult hospital malnutrition. JPEN J Parenter Enteral Nutr 2013;37:482-97. [Crossref] [PubMed]
- Itkin M, DeLegge MH, Fang JC, et al. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, with endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Gastroenterology 2011;141:742-65. [Crossref] [PubMed]
- Yolsuriyanwong K, Chand B. Update on endoscopic enteral access. Tech Gastrointest Endosc 2018;20:172-81. [Crossref]
- Pritchard C, Duffy S, Edington J, et al. Enteral nutrition and oral nutrition supplements: a review of the economics literature. JPEN J Parenter Enteral Nutr 2006;30:52-9. [Crossref] [PubMed]
- Braunschweig CL, Levy P, Sheean PM, et al. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr 2001;74:534-42. [Crossref] [PubMed]
- Gomes CA Jr, Andriolo RB, Bennett C, et al. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev 2015;CD008096. [PubMed]
- Grant JP. Comparison of percutaneous endoscopic gastrostomy with Stamm gastrostomy. Ann Surg 1988;207:598-603. [Crossref] [PubMed]
- Ho C-S, Yee AC, McPherson R. Complications of surgical and percutaneous nonendoscopic gastrostomy: review of 233 patients. Gastroenterology 1988;95:1206-10. [Crossref] [PubMed]
- Al-Abboodi Y, Ridha A, Fasullo M, et al. Risks of PEG tube placement in patients with cirrhosis-associated ascites. Clin Exp Gastroenterol 2017;10:211-4. [Crossref] [PubMed]
- Valentine RJ, Turner JW, Borman KR, et al. Does nasoenteral feeding afford adequate gastroduodenal stress prophylaxis? Crit Care Med 1986;14:599-601. [Crossref] [PubMed]
- Gauderer MW, Ponsky JL, Izant RJ. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg 1980;15:872-5. [Crossref] [PubMed]
- Acosta RD, Abraham NS, Chandrasekhara V, et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc 2016;83:3-16. [Crossref] [PubMed]
- Jain NK, Larson DE, Schroeder KW, et al. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy: a prospective, randomized, double-blind clinical trial. Ann Intern Med 1987;107:824-8. [Crossref] [PubMed]
- Lipp A, Lusardi G. Systemic antimicrobial prophylaxis for percutaneous endoscopic gastrostomy. Cochrane Database Syst Rev 2013;CD005571. [PubMed]
- Kwon RS, Banerjee S, Desilets D, et al. Enteral nutrition access devices. Gastrointest Endosc 2010;72:236-48. [Crossref] [PubMed]
- Takeshita N, Uesato M, Shuto K, et al. A 3-step gradual dilation method: a new safe technique of percutaneous endoscopic gastrostomy for obstructive esophageal cancer. Surg Laparosc Endosc Percutan Tech 2014;24:e140-2. [Crossref] [PubMed]
- Chadha KS, Thatikonda C, Schiff M, et al. Outcomes of percutaneous endoscopic gastrostomy tube placement using a T-fastener gastropexy device in head and neck and esophageal cancer patients. Nutr Clin Pract 2010;25:658-62. [Crossref] [PubMed]
- Yagishita A, Kakushima N, Tanaka M, et al. Percutaneous endoscopic gastrostomy using the direct method for aerodigestive cancer patients. Eur J Gastroenterol Hepatol 2012;24:77-81. [Crossref] [PubMed]
- Horiuchi A, Nakayama Y, Tanaka N, et al. Prospective randomized trial comparing the direct method using a 24 Fr bumper-button-type device with the pull method for percutaneous endoscopic gastrostomy. Endoscopy 2008;40:722-6. [Crossref] [PubMed]
- Maetani I, Tada T, Ukita T, et al. PEG with introducer or pull method: a prospective randomized comparison. Gastrointest Endosc 2003;57:837-41. [Crossref] [PubMed]
- Ginsberg GG. Direct percutaneous endoscopic jejunostomy. Tech Gastrointest Endosc 2001;3:42-9. [Crossref]
- Velázquez-Aviña J, Beyer R, Díaz-Tobar CP, et al. New method of direct percutaneous endoscopic jejunostomy tube placement using balloon-assisted enteroscopy with fluoroscopy. Dig Endosc 2015;27:317-22. [Crossref] [PubMed]
- Palmer LB, McClave SA, Bechtold ML, et al. Tips and tricks for deep jejunal enteral access: modifying techniques to maximize success. Curr Gastroenterol Rep 2014;16:409. [Crossref] [PubMed]
- Senadhi V, Chaudhary J, Dutta S. Percutaneous endoscopic gastrostomy placement during pregnancy in the critical care setting. Endoscopy 2010;42:E358-9. [Crossref] [PubMed]
- Savas N. Gastrointestinal endoscopy in pregnancy. World J Gastroenterol 2014;20:15241-52. [Crossref] [PubMed]
- Lee MJ, Saini S, Brink J, et al. Malignant small bowel obstruction and ascites: not a contraindication to percutaneous gastrostomy. Clin Radiol 1991;44:332-4. [Crossref] [PubMed]
- Oterdoom LH, Oterdoom DM, Ket JC, et al. Systematic review of ventricular peritoneal shunt and percutaneous endoscopic gastrostomy: a safe combination. J Neurosurg 2017;127:899-904. [Crossref] [PubMed]
- Vui HC, Lim WC, Law HL, et al. Percutaneous endoscopic gastrostomy in patients with ventriculoperitoneal shunt. Med J Malaysia 2013;68:389-92. [PubMed]
- Fein PA, Madane SJ, Jorden A, et al. Outcome of percutaneous endoscopic gastrostomy feeding in patients on peritoneal dialysis. Adv Perit Dial 2001;17:148-52. [PubMed]
- von Schnakenburg C, Feneberg R, Plank C, et al. Percutaneous endoscopic gastrostomy in children on peritoneal dialysis. Perit Dial Int 2006;26:69-77. [PubMed]
Cite this article as: Eguia E, Chand B. Endoscopic enteral access. Ann Laparosc Endosc Surg 2019;4:50.