Laparoscopic, robotic and transanal reversal of Hartmanns: pitfalls and challenges
Introduction
A Hartmann’s procedure (HP) was first introduced by a French Surgeon Dr. Henry Albert Hartmann in 1921 (1). It was initially described for the treatment of malignant colonic obstruction. Due to a high risk of anastomotic leak, the concept was to resect the offending pathology and form a colostomy, leaving a rectal stump in the pelvis. Although the primary pathology for a HP was for left sided colorectal cancer, this has been broaden to complicated diverticulitis (Hinchey grade III and IV), colonic trauma, and ischemic colitis (2,3). The avoidance of morbidity and mortality associated with anastomotic leak is key, as a temporising measure in the emergency setting (4) with the option reversing the colostomy at a stage where the risk profile is lower (5-7). Traditionally an open procedure, this is no longer the case with the advent use of minimally invasive technique (MIS). In a select group of patients requiring emergency HP, laparoscopic and robotic surgery can yield an equivalent morbidity and mortality rate compared to open procedure whilst preserving the benefits of MIS such as earlier return of normal bowel function, resumption of regular diet and less post-operative pain (7-9).
The first published report of a laparoscopically assisted Hartmann’s reversal was by Anderson et al. in 1993 (10) and since then there have been multiple studies showing laparoscopic technique a safe and feasible alternative to open Hartmann’s reversal (11-15). This was confirmed by a meta-analysis of level III evidence (16), which showed laparoscopic Hartmann’s procedure (LHR) was associated with less overall post-operative morbidity, length of stay, wound infection, and ileus. Patients with colostomies may have significant intra-abdominal adhesions, hence gaining access to the peritoneal cavity using a previous midline incision may increase likelihood of inadvertent bowel injury. Therefore the recommendation is to wait six months before a reversal is undertaken, however this can be shortened to three months in patients who will be favourable for a LHR (4,13).
LHR remains challenging in patients that are obese, those with narrow or frozen pelvis and fibrotic rectal stumps. Therefore, the conversion rate to open surgery can be up to 50% (16). The main reasons cited for conversion are adhesions, difficulty identifying the rectal stump or risk of injury to the rectum during its dissection (16). In the setting where the rectum have been divided below the peritoneal reflection and dense adhesions have occurred around the surrounding adjacent pelvic structures, a transanal total mesorectal excision (taTME) approach is a potential alternative as it allows for better visualization in virgin surgical territory with the additional pneumorectal dissection that helps open the plane (17).
Laparoscopic, robotic and transanal reversal of Hartmann’s procedure
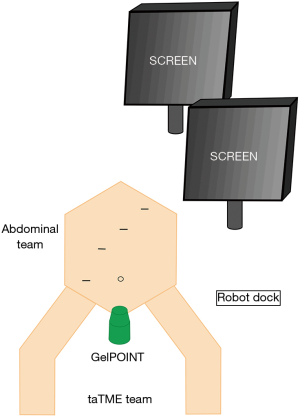
Patients received standardized bowel preparation day prior. The rectal stump was cleaned pre-operatively with an enema. All patients received antibiotic prophylaxis with 2g of cefazolin. Patients were fixed and placed in Lloyd Davies position with legs in padded, adjustable stirrups. Rectum was further irrigated with diluted iodine solution. A urinary catheter was placed under sterile conditions. Surgical team were organised into a two-team procedural approach (“Cecil”) as depicted in Figure 1.
Abdominal component
Standard OptiportTM entry was utilised and abdominal ports were placed with robotic arms positioned for a routine low anterior resection with the da Vinci® Si Surgical System. The taTME approach was set up synchronously. A pericolostomic incision was made and the colostomy was dissected free into the peritoneal cavity. We suggest a completion left colectomy to the level of the descending colon at a minimum. To achieve additional colonic length and a tension-free anastomosis the left colon can be mobilised by taking down the splenic flexure, in so doing, the inferior mesenteric vein may need to be ligated again. If further length is needed, the hepatic flexure can also be mobilized. A purse-string suture is placed in the proximal bowel and the anvil is secured into place.
Transanal component
A Lone Star retractor is placed and the rectal stump is identified, a Hegar dilator may be utilised if necessary. The transanal access platform is introduced through the anal canal with a 10 mm AirSeal trochar used to create pneumorectum and provide smoke evacuation. Initially an Olympus 0-degree camera is used with a CO2 pressure of 5 mmHg to create a planned mucosal mark with the use of hook diathermy. This was followed by full thickness circumferential rectotomy through both mucosal and muscle layers. Pressure was then increased to 12 mmHg and camera changed to 30-degree laparoscope. A flexible tip endoscope was used and the proximal part of the rectal stump was circumferentially dissected with diathermy, adjacent to the previous stapler line. Once advanced into the presacral space, the mesorectum is mobilised. This avascular plane of dissection is extended medially and laterally to enable circumferential rectal mobilisation. The peritoneal refection is then identified and divided to aid proximal stump removal, the resected tissue is then exteriorised through the anus. In patients with diverticular disease, it is important to ensure that the residual sigmoid colon is resected, at least, to the level of the rectosigmoid junction, we suggest resection to upper/mid rectum as it further reduces pressure on the anastomosis. The rectum is prepared for anastomosis with a circumferential prolene purse-string suture. The proximal colon with the attached anvil is connected to the circular stapler and an end-to-end anastomosis is performed within 10 cm of the anal verge. The anastomosis may be reinforced with sutures. The anastomosis position is confirmed laparoscopically, avoiding any tension. An air-leak test was performed to check anastomotic integrity. In our experience, without a loop ileostomy, healing rates of a tension free, well vascularised low pressure anastomosis with or without suture reinforcement is close to 100%.
We have utilised this technique in a patient with a long standing Crohn’s stump who was previously assessed, by two leading colorectal surgeons, as having a frozen pelvis abdominally and was deemed to have had a “permanent colostomy” for 20 years. Improvements in Crohn’s medical therapy meant his colonic health/colitis was better optimised pre-operatively. An open approach confirmed the presence of a frozen intraperitoneal pelvis. A synchronous transanal approach was utilised for this gentleman for a perineal primary approach to allow for restoration of intestinal continuity. He is now 2 years post reversal, and fully functional and working full time with good quality of life.
Pitfalls to reversal of Hartmann’s procedure
HP is usually performed in an emergency setting, often in medically co-morbid, frail and/or septic patients and is associated with relativity high morbidity and mortality. In current literature, reversal rates are variable but show a trend of being lower than 50% (18-20). Closure rates are higher if the HP is performed for a benign condition, such as perforated diverticulitis, as opposed to patients with a malignancy (21,22). This, at least in part, may be attributed to worse pre-morbid characteristics, need for further medical therapy and worse prognosis in patients with cancer.
Difficulty in reversal of Hartmann’s procedure can be classified into three stages: abdominal adhesiolysis, identification and dissection of rectal stump and performing the anastomosis. Previous peritonitis and its resultant adhesions can complicate not only entry into the peritoneal cavity but also subsequent colonic mobilization. Following Hartmann’s procedure, the rectal stump undergoes shrinkage and, with time, changes of diversion colitis (23). Several techniques have been described to help identify the rectal stump, some authors advocate implanting the rectal stump above the fascia and just under the skin during the original Hartmann’s procedure, this has the added benefit of minimizing the potential for rectal scarring and formation of small bowel adhesions around the stump. Others suggest the use of flexible sigmoidoscopy as it can assess for strictures, it noted, endoscopic balloon dilatation may be necessary. It is important to assess the health of the rectal stump prior to creating the anastomosis, if the end of the rectal stump is uneven, atrophic or fibrosed, this may need to be stapled off to ensure a safer anastomosis. Beware of proximal handsewn joins with long remnant stumps, there is a temptation to rejoin without further resection as they are matched, but, in our experience, we’ve found the pressure in these anastomoses along with the potential of anastomotic leakage to be much higher.
Tips and tricks for difficult reversal of Hartmann’s procedure
In extended left hemicolectomies, achieving a tension free anastomosis is often difficult as the residual colon is too short to reach the rectal stump. In such cases, we suggest performing a laparoscopic/or robotic retro-ileocolic colorectal anastomosis. Once flow is confirmed in the marginal artery, we advise ligation of the MCA high to aid with mobilizing the hepatic flexure and allowing the reflection of the right colon from the right paracolic gutter. Such a manoeuvre may be facilitated with a handport if difficult through conventional methods. A window is then created in an avascular area of the ileal mesentery underneath the ileocolic artery and the residual transverse colon is placed through the window to reach the anal canal, this reduces tension on the subsequent colorectal anastomosis.
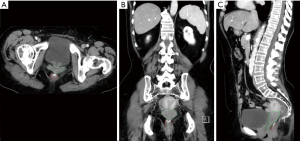
In very low colorectal anastomoses, we suggest ureteric stenting and in select cases where the stump is below the rectovaginal septum (Figure 2) we have had to perform an en-bloc hysterectomy to enable identification of the anorectal stump from above. If the rectal stump is truly extra-peritoneal, we suggest that the anorectal sphincter should be dissected out to find the remnants of the stump. Obviously in such cases this can be performed through an open, laparoscopic or robotic approach. If it is still difficult a transanal approach can be utilised easily, depending on surgeon familiarity.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.06.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sanderson ER. Henri Hartmann and the Hartmann operation. Arch Surg 1980;115:792-3. [Crossref] [PubMed]
- Rodkey GV, Welch CE. Surgical management of colonic diverticulitis with free perforation or abscess formation. Am J Surg 1969;117:265-9. [Crossref] [PubMed]
- ReMine SG. Dozois RR. Hartmann's procedure. Its use with complicated carcinomas of sigmoid colon and rectum. Arch Surg 1981;116:630-3. [Crossref] [PubMed]
- Fleming FJ, Gillen P. Reversal of Hartmann's procedure following acute diverticulitis: is timing everything? Int J Colorectal Dis 2009;24:1219-25. [Crossref] [PubMed]
- Pearce NW, Scott SD, Karran SJ. Timing and method of reversal of Hartmann's procedure. Br J Surg 1992;79:839-41. [Crossref] [PubMed]
- Vermeulen J, Coene PP, Van Hout NM, et al. Restoration of bowel continuity after surgery for acute perforated diverticulitis: should Hartmann's procedure be considered a one-stage procedure? Colorectal Dis 2009;11:619-24. [Crossref] [PubMed]
- Bell C, Asolati M, Hamilton E, et al. A comparison of complications associated with colostomy reversal versus ileostomy reversal. Am J Surg 2005;190:717-20. [Crossref] [PubMed]
- De'angelis N, Brunetti F, Memeo R, et al. Comparison between open and laparoscopic reversal of Hartmann's procedure for diverticulitis. World J Gastrointest Surg 2013;5:245-51. [Crossref] [PubMed]
- de'Angelis N, Felli E, Azoulay D, et al. Robotic-assisted reversal of Hartmann's procedure for diverticulitis. J Robot Surg 2014;8:381-3. [Crossref] [PubMed]
- Anderson CA, Fowler DL, White S, et al. Laparoscopic colostomy closure. Surg Laparosc Endosc 1993;3:69-72. [PubMed]
- Sosa JL, Sleeman D, Puente I, et al. Laparoscopic-assisted colostomy closure after Hartmann's procedure. Dis Colon Rectum 1994;37:149-52. [Crossref] [PubMed]
- Rosen MJ, Cobb WS, Kercher KW, et al. Laparoscopic restoration of intestinal continuity after Hartmann's procedure. Am J Surg 2005;189:670-4. [Crossref] [PubMed]
- Khaikin M. Laparoscopically assisted reversal of Hartmann's procedure. Surg Endosc 2007;21:1256. [Crossref] [PubMed]
- Haughn C, Ju B, Uchal M, et al. Complication rates after Hartmann's reversal: open vs. laparoscopic approach. Dis Colon Rectum 2008;51:1232-6. [Crossref] [PubMed]
- Yang PF, Morgan MJ. Laparoscopic versus open reversal of Hartmann's procedure: a retrospective review. ANZ J Surg 2014;84:965-9. [Crossref] [PubMed]
- Celentano V, Giglio MC, Bucci L. Laparoscopic versus open Hartmann's reversal: a systematic review and meta-analysis. Int J Colorectal Dis 2015;30:1603-15. [Crossref] [PubMed]
- Trépanier JS, Arroyave MC, Bravo R, et al. Transanal Hartmann's colostomy reversal assisted by laparoscopy: outcomes of the first 10 patients. Surg Endosc 2017;31:4981-7. [Crossref] [PubMed]
- Cellini C, Deeb AP, Sharma A, et al. Association between operative approach and complications in patients undergoing Hartmann's reversal. Br J Surg 2013;100:1094-9. [Crossref] [PubMed]
- Holland JC, Winter DC, Richardson D. Laparoscopically assisted reversal of Hartmann's procedure revisited. Surg Laparosc Endosc Percutan Tech 2002;12:291-4. [Crossref] [PubMed]
- Okolica D, Bishawi M, Karas JR, et al. Factors influencing postoperative adverse events after Hartmann's reversal. Colorectal Dis 2012;14:369-73. [Crossref] [PubMed]
- Seetharam S, Paige J, Horgan PG. Impact of socioeconomic deprivation and primary pathology on rate of reversal of Hartmann's procedure. Am J Surg 2003;186:154-7. [Crossref] [PubMed]
- Roque-Castellano C, Marchena-Gomez J, Hemmersbach-Miller M, et al. Analysis of the factors related to the decision of restoring intestinal continuity after Hartmann's procedure. Int J Colorectal Dis 2007;22:1091-6. [Crossref] [PubMed]
- Roe AM, Warren BF, Brodribb AJ, et al. Diversion colitis and involution of the defunctioned anorectum. Gut 1993;34:382-5. [Crossref] [PubMed]
Cite this article as: Gosavi R, Kong JCH, Warrier S. Laparoscopic, robotic and transanal reversal of Hartmanns: pitfalls and challenges. Ann Laparosc Endosc Surg 2019;4:58.