
Dilation and adjunct therapy for strictures
Introduction
Strictures can occur at any level of the gastrointestinal tract (GIT) from the esophagus to the colon. The causes of GIT strictures are numerous, such as reflux disease, congenital webs, caustic ingestion, malignancy, anastomotic, radiation, Schatzki’s rings, pyloric stenosis, nonsteroidal anti-inflammatory drug (NSAID) use, diverticulitis, or inflammatory bowel disease. In addition, these strictures are difficult to treat at times as each intervention may lead to further scarring and worsening stricture formation. As one may expect with such various etiologies and locations, there are many modalities of treatment. Historically, the first treatments involved dilation with fixed diameter bougies. As anesthesia and the field of surgery progressed, more invasive surgeries were developed that would allow for the resection of the diseased area. More recently, adjunctive endoscopic therapies such as stent placement, intralesional injections and electrosurgery have evolved to help solve this complex disease process.
Dilation
The earliest treatments were focused on the esophagus as it was the most accessible of areas. In the mid-1600s, Dr. Thomas Willis described using a thick sponge on the end of a whalebone bougie and in the 1700s, some doctors in Europe were recording the use of ivory bougies to dilate esophageal strictures (1). Over the past 150 years, various types of dilators have been used. Initially these were placed blindly, but fluoroscopic guidance has become a useful adjunct to aid with visualization during dilation. As the field of surgery progressed, retrograde dilators were able to be used by having the patient swallow a string which was then attached to a dilator through a gastrostomy site and pulled in a retrograde fashion through the esophagus. Another technique that has been used was using string guidance, whereby a patient swallowed a weighted string and after this passed into the distal esophagus past the stricture, GIT dilators were then passed over the taut string.
The most common blind bougie dilators in use today are the Hurst and Maloney dilators (Figure 1). Both are filled with tungsten and push type dilators that exert a shearing and radial force as they are pushed through a stricture. One point of differentiation with these two dilator types is that the Maloney dilators have a tapered type tip while the Hurst dilators have a blunt style tip. Another option that allows for slightly more control are Savary-Gilliard, American Dilation System and Tucker dilators that have an internal channel that allows for wire guidance. The Savary-Gilliard and American Dilation System dilators are push type dilators while the Tucker dilators are of the pull type. Tucker dilators have a loop on both tapered ends. They are pulled both antegrade and retrograde across a stricture. The Savary-Gilliard dilators have a radiopaque marking at the end of the taper point that is the maximum diameter whereas the American Dilation System dilators are totally radiopaque. Finally, anorectal Hegar dilators are stainless steel dilators with rounded ends. They are short and tend to be used for anorectal strictures. These dilators all have fixed maximum diameter and exert both radial and longitudinal shearing forces on the stricture during dilation.
In 1971, Lilly et al. reported the first endoscopic guided dilation which allowed a whole new avenue of therapies and began the use of balloon expandable dilators (2). These dilators come in various sizes ranging from 5 to 20 mm in diameter and exert only a radial force. By using the endoscope for guidance, it is possible to access strictures throughout the GIT as compared to the prior available dilators. They are designed to pass through the therapeutic channel of an endoscope with or without wire assistance. If inflated with radiopaque contrast, one is able to observe the dilation throughout the length of the stricture (Figure 2). The balloons are single use devices which can add to the cost of the procedure compared to bougie dilators. A handheld device to inject liquid is used to inflate the balloon, which then allows the proceduralist to monitor the pressure used during the dilation in addition to observing it through the endoscope. Over the years, a few trials have investigated comparing fixed diameter bougie dilators with endoscopic guided balloon dilators, mostly in the treatment of esophageal strictures as these are the most accessible to both treatment modalities. On a recent meta-analysis, no difference was noted in symptomatic relief, recurrence at one year, bleeding or perforation. However, patients who had balloon dilation did experience less severe post procedure pain (4).

Adjunct therapy
Although the majority of strictures can be effectively managed with endoscopic dilatation, additional measures can also be considered in the management of strictures depending on the etiology. Stent placement is one of the most common adjunctive modalities to maintain lumen patency, particularly with malignant strictures. Stents act by exerting radial force on the stricture; there are a variety of stents available depending on the location, length, material and diameter (Figure 3) (5).
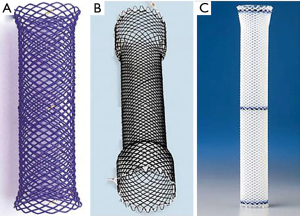
Uncovered stents are generally used in patients with poor survival and in areas with expected high likelihood of stent migration such as the duodenum and colon because of its benefit of flexibility to exert high pressure on angulated strictures in these regions (6). However, due to the risk of tumor and tissue in-growth with uncovered metal stents that can lead to re-obstruction and symptom recurrence, the majority of stents used for malignant strictures are either partially-covered or fully-covered metal designs, which have both been found to be equally effective and safe (7). Although fully-covered stents are more resistant to tumor and tissue in-growth, one of their disadvantages is that they are more prone to stent migration which can require re-intervention. Strategies to reduce the risk of stent migration include the use of large-sized stents and anchoring of the stents using clips at the stent margin or endoscopic sutures (8). Partially-covered stents do offer some of the advantages of both uncovered and fully covered stents; however, in benign pathologies, partially covered stents are not recommended due to the proliferation of granulation tissue through the proximal and distal uncovered stent edges which can make removal difficult. As such, fully-covered metal or self-expanding plastic stents are commonly used for benign strictures as a temporary treatment strategy which has the benefit that they are more easily removed compared to uncovered stents that can be more challenging to remove due to tissue ingrowth. To prevent excessive tissue in- or overgrowth, it is generally recommended that stents be removed by 6–10 weeks after treatment (9). Techniques for stent removal include using endoscopic rat tooth forceps to pull the purse-string suture at the proximal stent edge, double forcep graspers through a double-channel endoscope, stent-in-stent technique of temporary placement of a new stent within the first stent to facilitate removal and stent inversion of grasping the distal end of the stent and inverting it through itself. There is new evidence supporting the use of biodegradable stents which are typically made of polydioxanone that would not require removal; a recent multi-center randomized control trial found that biodegradable stent placement was associated with a significantly longer median time to first dilation with improved symptom control and higher level of activity compared to dilation alone with no difference in adverse events (10). However, further studies are still required to define the role of biodegradable stents in the treatment of benign strictures. Regardless of stent type, there was no significant difference found between stent types in the treatment of benign strictures, with an approximate success rate of 40% in benign esophageal strictures (11). The role of endoscopic stent placement will also be further discussed in another article in this series.
Endoscopic intralesional steroid injections have also been considered as an adjunctive therapy either before or after dilation and are typically used for refractory benign or anastomotic strictures. It is believed that intralesional steroids inhibit stricture formation by interfering with collagen synthesis, fibrosis and chronic scarring processes to help augment the effects of dilation but the mechanism of action is not entirely clear (12). The steroid utilized is typically triamcinolone acetate or acetonide in a concentration of 10–40 mg/mL injected using a 21 to 23 gauge 5 mm long sclerotherapy needle in 0.5 mL aliquots circumferentially in four quadrants at the proximal margin of the stricture as well as in the strictured segment when possible which should be difficult to inject if in the correct position (13). Although a few small studies demonstrated symptom improvement and an overall reduction in the need for repeat dilatations for benign esophageal strictures, these were not found to be statistically significant in a more recent meta-analysis. However, it was found to be consistent across all studies that there was a significant increase in the interval between dilations indicating some efficacy of intralesional steroids when used in combination with endoscopic dilation (14). The efficacy of intralesional steroids in addition to endoscopic balloon dilation was also found to be replicated with anastomotic strictures following esophagectomy in which a small randomized control trial demonstrated a significant reduction in the total number of dilations required as well as a significant improvement in re-stricture free survival (15).
With a similar logic as intralesional steroid injections that help counter collagen synthesis, fibrosis and scarring, people have investigated topical application of mitomycin C to strictures. In an animal study, mitomycin C in a dose dependent manner showed significant ability to prevent stricture formation after caustic esophageal injury with fairly good results which was then replicated in a case series. A double-blind, randomized, placebo controlled trial showed 80% resolution of esophageal strictures with topical mitomycin C treatment compared to only 35% in the placebo group as well as less total number of dilation sessions in the treatment group (16). Mitomycin C has also been injected intra-lesionally into the submucosal space in complex recurrent esophageal strictures, in which a study found that there was a significant improvement and resolution of dysphagia symptoms in the group treated with mitomycin C compared to the placebo group with no adverse effects (17). In addition, mitomycin C was found to be effective in stricture prevention in a porcine model (53.6% low dose, 35% high dose); however, in this trial, the esophageal wall treated with high-dose mitomycin C appeared to be necrotic and eventually led to perforation. In models treated with a low dose of mitomycin C, the esophageal wall appeared re-epithelialized and healthy (18).
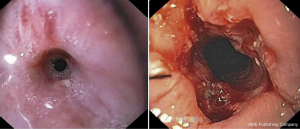
Another adjunctive measure to endoscopic dilations used for benign refractory and anastomotic strictures is electroincisional therapy in which electrocautery can be combined with argon plasma beam coagulation, needle-knife or endoscopic scissor techniques to incise the tissue of the stricture. The most common technique is the radial incision and cutting technique, in which the stricture is incised under direct endoscopic vison in a radial fashion parallel to the longitudinal axis to remove the rim of the stenosis (Figure 4) (19). The procedure is typically terminated once the scope can easily traverse the previously strictured segment (20). Incisional therapy can also be used in conjunction with dilation or steroid injections to reduce the need for repeated intervention. One randomized controlled trial found that electroincisional therapy was comparable in efficacy in terms of the total number of dilations and success rate when compared with Savary dilations with no increase in adverse events (19). Current evidence also suggests that incisional therapy is also most effective in the treatment of refractory benign or anastomotic strictures that have a relatively short stenosis less than 1cm in length with a success rate of approximately 80.6% and rate of recurrence of approximately 4.8% (21). As such, electroincisional therapy can be considered a safe and effective alternative method for the treatment of refractory strictures for whom multiple re-interventions have failed.
Complications
Given that the goal of dilation is to disrupt the stricture to enlarge the lumen, mucosal tears are expected. However, significant complications can occur which can include bleeding, perforation, bacteremia and aspiration which is mostly with upper GIT strictures and also related to sedation given for the procedure. Minor bleeding following dilation, stent insertion or electroincisional therapy is relatively common and typically self-limiting not requiring any intervention as fibrotic strictures are relatively avascular; however, significant bleeding from these interventions occurs at a rate between 0.1–0.4% (19,22). With this risk in mind, the American Society of Gastrointestinal Endoscopy (ASGE) came out with guidelines to help address the added bleeding risk of therapeutic endoscopy on patients who are on anti-coagulation or antithrombotics. For high-risk procedures such as dilation or incisional therapies, anti-coagulation and thienopyridines should be held for the appropriate time frame while non-steroidal anti-inflammatory drugs, aspirin and DVT prophylaxis should be continued even in high risk procedures. Controversially, endoluminal stent placement is categorized as low risk and as such, it is recommended to continue anti-coagulation in this setting (23). Perforation is the most clinically significant complication and can occur either from complete transmural disruption or creation of a false tract (24). It is estimated that the overall risk of perforation ranges from 0.1% to 0.6% (25). Factors that are associated with an increased risk of perforation include malignancy, severe inflammation, radiation or caustic-induced strictures as well as operator inexperience. Features of strictures that also increase the risk of perforation include long-segment, significant luminal narrowing or angulated strictures (26). Bacteremia is also a known complication from endoscopic dilation; however, it is rarely clinically significant as it is equivalent with brushing and flossing and the current ASGE guidelines do not recommend routine antibiotic prophylaxis at the time of endoscopic intervention for the management of strictures (27). Ultimately, endoscopic management of strictures is generally safe and well-tolerated. The benefits and risks of each technique must be weighed against other endoscopic alternatives, surgery or medical management and discussed thoroughly with patients.
Conclusions
Strictures can be located throughout the GIT and can be successfully treated using endoscopic techniques such as dilation, stents, and intralesional injections with either steroids or mitomycin C or electroincisional therapy. Careful consideration should be made to characterize the stricture prior to intervention and the method used for the treatment of strictures is dependent on etiology, location, length and degree of stenosis. Each option can be repeated if necessary and used in a step-wise approach to relieve symptoms and improve the success of intervention. Overall, endoscopic management of GIT strictures is relatively safe, effective and has a favorable risk-benefit profile. Additional studies are required to better define the long-term efficacy of endoscopic options such as intralesional steroid or mitomycin C injections, biodegradable stents and electroincisional therapy.
Acknowledgments
The authors would like to thank Ryan Juza and Jeffery Marks for allowing us to participate in this special issue on surgical endoscopy.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jeffrey M. Marks and Ryan M. Juza) for the series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.06.06). The series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kravetz RE. Eder-Puestow “stringless” dilators. Am J Gastroenterol 2009;104:1631-2. [Crossref] [PubMed]
- Lilly JO, McCaffery TD. Esophageal stricture dilatation. A new method adapted to the fiberoptic esophagoscope. Am J Dig Dis 1971;16:1137-40. [Crossref] [PubMed]
- Johnson R, Fung EC. Depiction of a fluoroscopic-guided balloon dilation of a pyloric stricture with the characteristic “waist” indicating the location of the stricture followed by further balloon dilation until the waist and stricture disappear. Asvide 2019;6:192. Available online: http://www.asvide.com/article/view/32545
- Josino IR, Madruga-Neto AC, Ribeiro IB, et al. Endoscopic Dilation with Bougies versus Balloon Dilation in Esophageal Benign Strictures: Systematic Review and Meta-Analysis. Gastroenterol Res Pract 2018;2018:5874870 [Crossref] [PubMed]
- Bechara R, Inoue H. Recent advancement of therapeutic endoscopy in esophageal benign disease. World J Gastrointest Endosc 2015;7:481-95. [Crossref] [PubMed]
- Kochar R, Shah N. Enteral stents: from esophagus to colon. Gastrointest Endosc 2013;78:913-8. [Crossref] [PubMed]
- McGaw C, Alkaddour A, Vega KJ, et al. Stent type used does not impact complication rate or placement time but can decrease treatment cost for benign and malignant esophageal lesions. World J Gastrointest Endosc 2016;8:338-43. [Crossref] [PubMed]
- Vanbiervliet G, Filippi J, Karimdjee BS, et al. The role of clips in preventing migration of fully covered metallic esophageal stents: a pilot comparative study. Surg Endosc 2012;26:53-9. [Crossref] [PubMed]
- van Heel NC, Haringsma J, Spaander MC, et al. Short-term esophageal stenting in the management of benign perforations. Am J Gastroenterol 2010;105:1515-20. [Crossref] [PubMed]
- Walter D, van den Berg MW, Hirdes MM, et al. Dilation or biodegradable stent placement for recurrent benign esophageal strictures: a randomized controlled trial. Endoscopy 2018;50:C12. [Crossref] [PubMed]
- Fuccio L, Hassan C, Frazzoni L, et al. Clinical outcomes following stent placement in refractory benign esophageal stricture: a systematic review and meta-analysis. Endoscopy 2016;48:141-8. [PubMed]
- Groth SS, Odell DD, Luketich JD. Esophageal Strictures Refractory to Endoscopic Dilatation. In: Pawlik T, Maithel SK, Merchant NB. editors. Gastrointestinal Surgery: Management of Complex Perioperative Complications. New York: Springer-Verlag, 2015:13-22.
- Kochhar R, Makharia GK. Usefulness of intralesional triamcinolone in treatment of benign esophageal strictures. Gastrointestinal Endoscopy 2002;56:829-34. [Crossref] [PubMed]
- Szapáry L, Tinusz B, Farkas N, et al. Intralesional steroid is beneficial in benign refractory esophageal strictures: A meta-analysis. World J Gastroenterol 2018;24:2311-9. [Crossref] [PubMed]
- Hanaoka N, Ishihara R, Motoori M, et al. Endoscopic Balloon Dilation Followed by Intralesional Steroid Injection for Anastomotic Strictures After Esophagectomy: A Randomized Controlled Trial. Am J Gastroenterol 2018;113:1468-74. [Crossref] [PubMed]
- El-Asmar KM, Hassan MA, Abdelkader HM, et al. Topical mitomycin C application is effective in management of localized caustic esophageal stricture: A double-blinded, randomized, placebo-controlled trial. J Pediatr Surg 2013;48:1621-7. [Crossref] [PubMed]
- Machida H, Tominaga K, Minamino H, et al. Locoregional mitomycin C injection for esophageal stricture after endoscopic submucosal dissection. Endoscopy 2012;44:622-5. [Crossref] [PubMed]
- Wu Y, Schomisch S, Cipriano C, et al. Preliminary results of antiscarring therapy in the prevention of postendoscopic esophageal mucosectomy strictures. Surg Endosc 2014;28:447-55. [Crossref] [PubMed]
- Hordijk ML, van Hooft JE, Hansen BE, et al. A randomized comparison of electrocautery incision with Savary bougienage for relief of anastomotic gastroesophageal strictures. Gastrointest Endosc 2009;70:849-55. [Crossref] [PubMed]
- Samanta J, Dhaka N, Sinha SK, et al. Endoscopic incisional therapy for benign esophageal strictures: Technique and results. World J Gastrointest Endosc 2015;7:1318-26. [Crossref] [PubMed]
- Lee TH, Lee SH, Park JY, et al. Primary incisional therapy with a modified method for patients with benign anastomotic esophageal stricture. Gastrointest Endosc 2009;69:1029-33. [Crossref] [PubMed]
- Pereira-Lima JC, Ramires RP, Zamin IJ, et al. Endoscopic dilation of benign esophageal strictures: report on 1043 procedures. Am J Gastroenterol 1999;94:1497-501. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc 2016;83:3-16. [Crossref] [PubMed]
- ASGE Technology Committee. Tools for endoscopic stricture dilation. Gastrointest Endosc 2013;78:391-404. [Crossref] [PubMed]
- Lew RJ, Kochman ML. A review of endoscopic methods of esophageal dilation. J Clin Gastroenterol 2002;35:117-26. [Crossref] [PubMed]
- Irani S, Kozarek RA. Techniques and principles of endoscopic treatment of benign gastrointestinal strictures. Curr Opin Gastroenterol 2015;31:339-50. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2015;81:81-9. [Crossref] [PubMed]
Cite this article as: Johnson R, Fung EC. Dilation and adjunct therapy for strictures. Ann Laparosc Endosc Surg 2019;4:63.



