
Surgeons performing endoscopy: why, how, and when?
History of endoscopy: the answer to why
The desire to examine the organs of the human body through natural orifices is not new (1). The history of endoscopy dates back to Hippocrates (460–375 BC), when he described the use of a rectal speculum similar to those currently in use. Similar instruments were described in Roman medicine, including a three bladed vaginal speculum found in the ruins of Pompeii. Albulassim (912 AD) reflected light with mirror to view into body cavities, and Guilio Cesare Aranzi, a Venezuelan, used Camera Obscura [1587] to focus a ray of light to permit viewing the nasal cavity. Bozzini [1806] presented his Lichtleiter, or light conductor, to the Faculty of Medicine in Vienna. There was no evidence that his instrument ever was used on a human subject and Bozzini, now recognized as a visionary, was met with ridicule in Vienna for proposing this early endoscopic device. The instrument was thought to be dangerous and too painful for clinical use. Although further development of Bozzini’s Lichtleiter was halted, he had laid the foundation for others to build upon. Segalas [1826] used the principles demonstrated in Bozzini’s Lichtleiter to develop the speculum urethro-cystique, which utilized two small candles and a conical mirror to focus light into the bladder, allowing visual inspection. In 1853, Antonin J. Desormeaux adapted a kerosene lamp burning alcohol and turpentine held in a reservoir at the base of the handle, to generate a narrower beam of light that was thought to achieve brighter illumination of the area to be visualized. Desormeaux is often referred to as the Father of Cystoscopy. Desormeaux was the first physician to use the Lichtleiter in a patient, and he was the first to use the term Endoscopy and also to use endoscopic instruments for diagnostic and therapeutic purposes (2). Kussmual performed the first successful gastroscopy in 1886. Leiter developed an esophagoscope with a mignon lamp in 1881, and Chevalier Jackson, an Otolaryngologist, first used the bronchoesophagoscope to remove foreign bodies from humans in 1907. In 1937, Rudolf Schindler developed the semi flexible endoscope, and in 1968, McCune, Shorb, and Moscovitz described the first successful endoscopic cannulation of the pancreatic and bile ducts, spurring enthusiasm for endoscopic retrograde cholangiopancreatography (ERCP). Wolf & Shinya performed the first colonic polypectomy using a flexible endoscope in 1974. Ponsky and Gauderer revolutionized the nutritional care of patients by describing the percutaneous endoscopic gastrostomy (PEG) in 1980, and Steigmann and Goff described endoscopic variceal band ligation in 1988. The history of surgery is rich with innovation, and the evolution of flexible endoscopy as a valuable clinical tool largely has been led by surgeons sharing their insights with the world (Table 1). Additionally to the founding role that many prominent surgeons played in the establishment of endoscopy as a diagnostic and therapeutic approach to patient care, surgeon endoscopists are an important element of the healthcare workforce (3). The burden of screening procedures alone already outstrips the labor supply in most areas, and without the contribution of surgeons skilled in performing screening, therapeutic, curative, and palliative endoscopic procedures, patient access to appropriate care would be severely limited. Surgeons must perform endoscopy to benefit their patients, and to advance their specialty.
Table 1
| Procedure | Surgeon |
|---|---|
| Colonoscopy | Turell |
| Control of hemorrhage | Gaisford, Sugawa |
| Polypectomy | Shinya, Wolf |
| ERCP | McCune, Shorb |
| Variceal banding | Steigman |
| PEG | Ponsky, Gauderer |
| Biliary stenting | Sohendra |
ERCP, endoscopic retrograde cholangiopancreatography; PEG, percutaneous endoscopic gastrostomy.
A study comparing the practice patterns of rural vs. urban surgeons showed that both groups perform endoscopy as a routine part of their practices but rural surgeons perform endoscopy in significantly higher numbers (4). A prospective analysis of 3,525 EGDs performed by surgeons published in Surgical Endoscopy showed that surgeons perform EGD with excellent outcomes. No complications were reported in 3,447 patients (97.8%) (5). A prospective analysis of 13,580 colonoscopies performed by surgeons demonstrated superb outcomes, with a 0.2% complication rate and 0.007% perforation rate reported (6). Lee et al. demonstrated equivalent adenoma detection rates (ADR) between surgeons and gastroenterologists, adding to the body of literature demonstrating that surgeon endoscopists are careful experts with excellent outcomes serving the needs of their patients (7).
Training in flexible endoscopy: the answer to how
Flexible endoscopy is one of the main tools of the practicing surgeon. The American Board of Surgery (ABS) recognized the increasing role of endoscopy in surgical practice, and in 1985, recommended a minimum of 29 endoscopic cases to be performed by graduating surgical residents. This was based on the intent to ensure that all surgeons had exposure to endoscopic techniques. In response to the argument that this experience set the threshold too low for a graduating resident to be competent in endoscopy, this requirement later was modified. Effective with residents graduating in June 2009 (8), the requirement was modified to include 35 EGD and 50 colonoscopies as new minimum threshold values.
The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), a professional society founded on the principles of surgeon performed endoscopy, developed the Fundamentals of Endoscopic Surgery (FES) program as a competency-based platform for training in endoscopy. The FES curriculum, a robust collection of didactic materials is made available free of charge to all interested in learning the practice of endoscopy regardless of specialty and has been incorporated into the general surgery residency curriculum. Additionally, successful FES certification now is a requirement for a resident to sit for the ABS Qualifying Examination. In full display of its support for the FES Program, the ABS Certifying Examination now includes more scenarios related to the use of flexible endoscopy in the care of patients with gastrointestinal diseases. The principles of competency-based education that historically have hallmarked surgical training include training in flexible endoscopy, in recognition of its important place in the care of surgical patients. For surgeons planning to make flexible endoscopy a central part of their practices, or for those who did not garner as robust an experience with these techniques as they desired, many fellowship opportunities have been created. Opportunities exist for practicing surgeons to augment their endoscopic skills, but given that the future of general surgery will in part be based on the flexible endoscopy platform, those in training are encouraged to take every opportunity to master this essential approach.
Performing flexible endoscopy: the answer to when
Surgeons use endoscopy very often in the preoperative, intraoperative, and postoperative care of their patients. In the preoperative period, endoscopy mainly is used to diagnose conditions and localize lesions, for operative planning, and to provide nutritional support (9). Intraoperative endoscopy is used to localize lesions, evaluate the anatomic result of surgery, and to test for anastomotic integrity during foregut and colorectal reconstructive procedures. The real-time feedback provided during fundoplication, esophageal myotomy, and obesity surgery as examples serves to guide the remaining portions of the operation. Postoperative endoscopy fulfills an important clinical role in follow up for patients with premalignant and malignant conditions, like Barrett esophagus, colonic polyps, and post-treatment surveillance needs after resection for esophageal, gastric, and colon cancer as three examples. Flexible endoscopy plays an important role in the management of operative complications as well, including marginal ulcers, anastomotic leaks, fistulas, and retained biliary stones.
Although traditionally the focus of the flexible endoscopy platform has been on diagnostic and therapeutic interventions that now are commonplace, like polypectomy, numerous advanced techniques have been developed that squarely will influence surgical practice going forward. A few specific areas that form the basis of future endoluminal surgical approaches are featured below.
Peroral endoscopic myotomy (POEM) for esophageal achalasia and delayed gastric emptying
POEM was first described in 2007 in a porcine model, and now 12 years later, more than 5,000 cases have been performed worldwide for esophageal achalasia (10,11). When performed by experienced endoscopists, POEM is safe and effective (11,12). Multicenter comparisons between POEM and laparoscopic Heller myotomy (LHM) have demonstrated that POEM more frequently results in a significant clinical response compared to LHM (98.0 % vs. 80.8 %; P=0.01), exhibits a shorter mean procedure time than LHM (102 vs. 264 min; P<0.01), and provides a longer length of myotomy (16 vs. 8 cm; P< 0.01). POEM has a favorable adverse event rate as compared to LHM as well (6 % vs. 27 %; P<0.01) (13). Within a decade, POEM has emerged as the technique of choice in the treatment of patients with achalasia, and it serves as a prime example of how rapidly the surgical landscape will change as more disruptive endoscopic approaches are adopted going forward. There are many examples of surgical procedures that already have been replaced by newer techniques based on the flexible endoscopy platform (Table 2).
Table 2
| Surgical procedure | Endoscopic procedure replacing surgery |
|---|---|
| Esophagus | |
| Foreign body removal | Endoscopic foreign body removal |
| Stricture management | Endoluminal stents or balloon dilation |
| Palliation of malignancy | Endoluminal stents or balloon dilation |
| Variceal bleeding | Endoscopic variceal banding |
| Achalasia | Balloon dilation, PEOM instead of Heller myotomy |
| Barrett esophagus | RF ablation; endoscopic mucosal resection |
| Perforation | Endoluminal clip application for closure of perforation |
| Stomach | |
| Upper GI bleeding | Endoscopic clipping, injection, cauterization |
| Pyloric obstruction | Endoscopic stents, balloon dilation |
| Foreign body | Endoscopic foreign body removal |
| Enteral feeding access | PEG, PEJ, GJ tube placement |
| Operative fundoplication (GERD) | TIF, Stretta |
| Obesity | Bariatric endoscopy |
| Pancreaticobiliary | |
| Common bile duct stones | ERCP |
| Biliary strictures | Endoscopic stents, balloon dilation |
| Pancreatic pseudocysts | Endoscopic cystogastrostomy |
| WOPN | Endoscopic transgastric drainage |
| Acute cholecystitis | Cholecystoduodenostomy |
| Small intestine and colon | |
| Enteral access | PEG, PEJ, endoscopic GJ tube placement |
| Stricture | Endoscopic dilation of stricture |
| Lower GI bleeding | Endoscopic clipping, injection, suturing, hemostatic spray, cauterization |
| Colonic stricture | Endoscopic stents, balloon dilation |
| Hemorrhoid disease | Endoscopic band ligation |
| Colonic polyps | Endoscopic polypectomy |
POEM, peroral endoscopic myotomy; GI, gastrointestinal; PEG, percutaneous endoscopic gastrostomy; PEJ, percutaneous jejunostomy; GJ, gastrojejunal; TIF, transoral incisionless fundoplication; GERD, gastroesophageal reflux disease; ERCP, endoscopic retrograde cholangiopancreatography; WOPN, walled off pancreatic necrosis.
While achalasia may remain underdiagnosed, it still is uncommon compared with many other disease processes we treat as surgeons and endoscopists. Gastroparesis, however, is on the rise and the advent of G-POEM, endoscopic myotomy aimed at dividing the pyloric musculature to improve gastric emptying, has both improved patient care and introduced an area where appropriately skilled surgeons may be relevant in this disease process (14,15).
Transoral incisionless fundoplication (TIF)
The first TIF using the EsophyX device was performed (Redmond, Washington, USA) in 2005 (16). Since then, TIF has emerged as an effective alternative in the management of GERD in patients with intact anatomy, a response to pharmacologic therapy, and who have not developed Barrett esophagus. Multicenter randomized controlled trials have confirmed its safety and effectiveness, and heave demonstrated its superiority over proton-pump inhibitors (PPIs) in selected patient populations with incomplete symptomatic response to maximal medical therapy (17,18). GERD impacts much of the world’s population, and effective treatments devoid of the concerns associated with the use of acid suppressing medications will serve the needs of our patients well. Combining these attributes with outpatient treatment delivery based on the flexible endoscopy platform makes it clear that our future role as surgeons in the treatment of GERD will require robust endoscopy skills if we are to remain of service to our patients.
Submucosal tunneling endoscopic resection (STER)
STER was described first in 2012 as a novel method for the endoscopic resection of mural lesions of the GI tract arising from the muscularis propria (19). It has compared favorably with endoscopic full-thickness resection (EFTR) in that the number of endoscopic clips required is reduced, the amount of time spent suturing is reduced, and when performed in hospitalized patients, the length of stay is reduced (20). Although this is not a procedure that most surgeons will find useful in their daily practices at present, this represents yet another disruptive endoscopic approach to surgical procedures that one day will supplant, or replace, surgical treatment for intramural lesions of the GI tract (Figure 1). STER, and the other burgeoning approaches to intramural, or third space endoscopy, serves as a reminder to surgeons that the development and continual refinement of their endoscopic skill set is of paramount importance to their patients and to the future of our specialty.
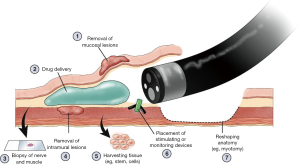
The future of surgeon performed endoscopy
Thirty years ago, the advent of operative laparoscopy ushered in a then new era in the field of general surgery. Operative procedures underwent wholesale change, and new procedures were developed, leveraging the strengths of the laparoscopic platform. Hospital dynamics were disrupted as patients who once remained hospitalized for a week now were treated as outpatients. Robotic technology, in some fields, have furthered these advances.
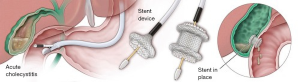
The future of surgery will be influenced by the future of surgeon performed endoscopy. The frontiers of third space endoscopy or intramural endoscopy will continue to advance. Device enhancements, like lumen apposing metal stents (LAMS), will drive procedures from combined laparoscopic-endoscopic approaches to entirely endoscopic techniques, further reducing their invasiveness. One example is the treatment of choledocholithiasis following gastric bypass surgery. Once requiring laparotomy and open common bile duct exploration, this clinical circumstance routinely has been treated using laparoscopic access to the remnant stomach with concomitant transabdominal ERCP during recent years. It is now becoming more commonplace to utilize LAMS to provide a path from the alimentary limb through the remnant stomach so that more traditional ERCP may be offered the patient, without the need for surgical exposure (21,22). LAMS also are being utilized in cases of acute cholecystitis in those too infirm to undergo cholecystectomy, as an effective and more durable alternative to cholecystostomy placement (23,24) (Figure 2). As we continue to seek increasingly less invasive approaches to patient care, surgery will evolve, and the flexible endoscope is the platform central to our next phase of evolution as surgeons.
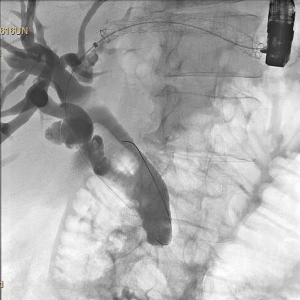
Endoscopic ultrasound (EUS) is an advanced endoscopic imaging technique that will open up new avenues of patient care that will become important in the future of surgeon performed endoscopy, and in general surgery. EUS is a valuable imaging modality that already has a wide range of diagnostic and therapeutic applications. Already an established adjunct for diagnosis and staging of GI malignancies, novel applications of EUS continually are evolving (25). Contrast enhanced EUS (CE-EUS) is proving useful in several GI conditions, and elastography will expand the diagnostic utility of EUS for solid lesions. EUS serves as a robust platform for therapeutic measures though, and that will continue to increase in value for surgeons who embrace the endoscopic platform as the foundation for their future. As one example, EUS directed hepaticogastrostomy is useful in the palliative treatment of malignant biliary obstruction for patients in whom ERCP has failed (Figure 3). This approach spares patients the need for external drainage devices typical of those placed by our interventional radiology colleagues, and preserves their mobility, dignity, and quality of life.
Conclusions
The role of the surgeon is to innovate. The field of endoscopy largely represents surgical innovation, and we must continue to expand the utility of the flexible endoscopy platform as it forms the basis for the future of general and gastrointestinal surgery. Surgeon performed endoscopy is an essential component of surgical practice, and is a set of skills that will be critical in our quest to treat patients using increasingly less invasive approaches. We are not surgical endoscopists; we are surgeons who perform diagnostic and therapeutic endoscopy, and endoscopic surgery. The endoscope is one of our many tools, essential as any other.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jeffrey M. Marks and Ryan M. Juza) for the series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.06.09). The series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately addressed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zetka JR. Surgeons and the scope. Ithaca, NY: ILR Press, 2003.
- Leger P. Antonin Jean Desormeaux. Prog Urol 2004;14:1231-8. [PubMed]
- Kocher R, Sahni NR. Rethinking Health Care Labor. N Engl J Med 2011;365:1370-2. [Crossref] [PubMed]
- Heneghan SJ, Bordley IV J, Dietz PA, et al. Comparison of urban and rural general surgeons: motivations for practice location, practice patterns, and education requirements. J Am Coll Surg 2005;201:732-6. [Crossref] [PubMed]
- Reed WP, Kilkenny JW, Dias CE, et al. A prospective analysis of 3525 esophagogastroduodenoscopies performed by surgeons. Surg Endosc 2004;18:11-21. [Crossref] [PubMed]
- Wexner SD, Garbus JE, Singh JJ. A prospective analysis of 13,580 colonoscopies. Reevaluation of credentialing guidelines. Surg Endosc 2001;15:251-61. [Crossref] [PubMed]
- Lee AHH, Lojanapiwat N, Balakrishnan V, et al. Is there a difference in adenoma detection rates between gastroenterologists and surgeons? World J Gastrointest Endosc 2018;10:109-16. [Crossref] [PubMed]
- Britt LD, Richardson JD. Residency review committee for surgery: an update. Archives of Surgery 2007;142:573-5. [Crossref] [PubMed]
- Martinez J, Akpinar E, Andres Astudillo J. Use of endoscopy to prepare patients for surgery. Tech Gastrointest Endosc 2013;15:173-9. [Crossref]
- Pasricha PJ, Hawari R, Ahmed I, et al. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy 2007;39:761-4. [Crossref] [PubMed]
- Stavropoulos SN, Desilets DJ, Fuchs KH, et al. Per-oral endoscopic myotomy white paper summary. Surg Endosc 2014;28:2005-19. [Crossref] [PubMed]
- Swanstrom LL, Kurian A, Dunst CM, et al. Long-term outcomes of an endoscopic myotomy for achalasia: the POEM procedure. Ann Surg 2012;256:659-67. [Crossref] [PubMed]
- Kumbhari V, Tieu AH, Onimaru M, et al. Peroral endoscopic myotomy (POEM) vs laparoscopic Heller myotomy (LHM) for the treatment of Type III achalasia in 75 patients: a multicenter comparative study. Endosc Int Open 2015;3:E195-201. [Crossref] [PubMed]
- Chung H, Khashab MA. Gastric Peroral Endoscopic Myotomy. Clin Endosc 2018;51:28-32. [Crossref] [PubMed]
- Dacha S, Mekaroonkamol P, Li L, et al. Outcomes and quality-of-life assessment after gastric per-oral endoscopic pyloromyotomy (with video). Gastrointest Endosc 2017;86:282-9. [Crossref] [PubMed]
- Cadiere GB, Rajan A, Rqibate M, et al. Endoluminal fundoplication (ELF)--evolution of EsophyX, a new surgical device for transoral surgery. Minim Invasive Ther Allied Technol 2006;15:348-55. [Crossref] [PubMed]
- Hunter JG, Kahrilas PJ, Bell RC, et al. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology 2015;148:324-33.e5. [Crossref] [PubMed]
- Hakansson B, Montgomery M, Cadiere GB, et al. Randomised clinical trial: transoral incisionless fundoplication vs. sham intervention to control chronic GERD. Aliment Pharmacol Ther 2015;42:1261-70. [Crossref] [PubMed]
- Xu MD, Cai MY, Zhou PH, et al. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 2012;75:195-9. [Crossref] [PubMed]
- Duan TY, Tan YY, Wang XH, et al. A comparison of submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric fundus submucosal tumors. Rev Esp Enferm Dig 2018;110:160-5. [PubMed]
- Baron TH, Song LM, Ferreira LE, et al. Novel approach to therapeutic ERCP after long-limb Roux-en-Y gastric bypass surgery using transgastric self-expandable metal stents: experimental outcomes and first human case study (with videos). Gastrointest Endosc 2012;75:1258-63. [Crossref] [PubMed]
- Shakhatreh MH, Yeaton P. ERCP through a gastrojejunal lumen-apposing stent. VideoGIE 2016;1:19-21. [Crossref] [PubMed]
- Kalva NR, Vanar V, Forcione D, et al. Efficacy and Safety of Lumen Apposing Self-Expandable Metal Stents for EUS Guided Cholecystostomy: A Meta-Analysis and Systematic Review. Can J Gastroenterol Hepatol 2018;2018:7070961 [Crossref] [PubMed]
- Jain D, Bhandari BS, Agrawal N, et al. Endoscopic Ultrasound-Guided Gallbladder Drainage Using a Lumen-Apposing Metal Stent for Acute Cholecystitis: A Systematic Review. Clin Endosc 2018;51:450-62. [Crossref] [PubMed]
- Cartana ET, Parvu D, Saftoiu A. Endoscopic ultrasound: current role and future perspectives in managing rectal cancer patients. J Gastrointestin Liver Dis 2011;20:407-13. [PubMed]
Cite this article as: Fanelli RD, Sultany MS. Surgeons performing endoscopy: why, how, and when? Ann Laparosc Endosc Surg 2019;4:66.


