
The effect of vascular anatomy and gender on bowel function after right colectomy with extended D3-mesenterectomy
Introduction
The incidence of colon cancer is on the rise, with the highest incidence in Australia, New Zealand, Canada, the United States, and parts of Europe. Despite improved surgery and adjuvant chemotherapy, the mortality lies between 30% and 40%, in some regions even higher. According to the estimates by International Agency for Cancer Research, the global incidence of colon cancer is 1,096,601. Worldwide the estimated annual number of deaths related to colon cancer is 551,269 (1). Colon cancer tends to spread along the lymphatic vessels to the lymph nodes. To improve survival rates, surgeons increasingly focus on more extensive lymphadenectomy during surgery for colon cancer. Cancer in the right colon represents a particular challenge. The neurovascular stem of the midgut—made up of the superior mesenteric vessels with the surrounding nerves and lymphatics—irrigates both the right colon and the entire small bowel. Due to such anatomy, only relatively peripheral branches of the superior mesenteric artery (SMA) supplying the right colon can be included in the mesenterectomy. Traditionally only the lymph nodes around these branches have been removed whereas the lymph nodes surrounding the common stem have remained untouched. Surgeons have now developed a technique to safely remove all the tissue—including the lymph nodes—around the superior mesenteric vessels up to the pancreatic notch without injuring the blood vessels (2). As a consequence of this extensive mesenterectomy, the superior mesenteric nerve plexus (SMP) at this level is transected (3).
In a previous study we found a small, but significant increase in stool frequency of 0.48 stools per day in patients who had undergone right colectomy with this extensive mesenterectomy (also referred to as extended D3-mesenterectomy), compared to the patients who had undergone the traditional right colectomy without the nerve transection (D2-mesenterectomy) (4). In the study mentioned above, most of the denervated patients had one or more jejunal arteries arising proximal to the middle colic artery (i.e., proximal to the level of dissection). They seemed to have a better postoperative bowel function than the patients without such arteries. Our hypothesis was that nerves following these arteries spared the innervation of the small intestine through branches accompanying the arcades in the mesentery (Figure 1). The number of subjects without jejunal artery(ies) cranial to the middle colic artery was too small to conclude. In addition, the impression that there is a gender-related difference concerning the postoperative bowel function after extended D3-mesenterectomy remained to be proved.
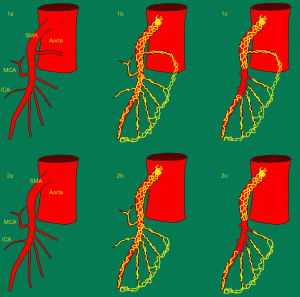
The aim of this study is to examine how the number of arteries proximal to the level of dissection affects the long-term postoperative bowel function in a larger group of patients undergoing right colectomy with extended D3-mesenterectomy. A secondary aim is to examine the effect of gender on the postoperative bowel function in the same patients. Furthermore, we want to see if the normalization of the bowel function seen in the previous study, continues when the observation time is prolonged.
Methods
Patients
Experimental group
Consecutive patients included in the prospective trial “Safe Radical D3 Right Colectomy for Cancer through preoperative Biphasic Multi-detector Computed Tomography” [2012–2018].
Control group
Patients operated with right colectomy for cancer without extended mesenterectomy (i.e., D2) [2007–2018].
Inclusion criteria
Patients, with right-sided colon cancer (from cecum to the proximal transverse colon), undergoing right colectomy with intent to cure.
Exclusion criteria
To increase the external validity of the outcome, we excluded patients with known disorders (e.g., celiac disease, inflammatory bowel disease), patients with extended bowel resection, untreatable recurrence, and patients having adjuvant chemotherapy less than 6 months prior to the interview. We also excluded patients with a follow-up shorter than 6 months (the minimal time we assumed the bowel would need to adapt after surgery) (5). Further exclusion criteria were refusal to participate, dementia, and death.
Anatomy
A 3D anatomical map of the superior mesenteric artery and vein (SMA and SMV), with all their branches, was derived from the CT scan taken before surgery using the FDA-approved Osirix MD v. 10.0.2 64-bit image processing application (Pixmeo, Bernex, Switzerland), Mimics medical image processing software ver. 21.0.0.406, and 3-matic medical software ver. 13.0.0.188, both Windows 7 ultimate edition x64 2018 (both Materialize NV, Leuven, Belgium). This roadmap was used during the operation. It also provided information about the number of jejunal arteries (JA) arising proximal to the root of the middle colic artery (MCA) (i.e., the number of arterial branches arising proximal the SMP transection).
Surgical procedure
The surgical procedure has been described in previous articles (2). The mesenterectomy involves complete removal of the tissue, including the vascular sheaths, anterior and posterior to the SMA and SMV from 0.5 cm proximal to the root of the MCA (at the pancreatic notch) to 1 cm distal to the root of the ICA. The medial border follows the left margin of SMA. The surgical approach is medial-to-lateral and en bloc.
The instruments used
Diarrhea Assessment Scale (DAS): a validated tool for grading four manifestations of diarrhea (frequency, consistency, urgency and abdominal discomfort) in the context of oncologic therapy with a scale from 0 to 12, where 12 represents severe diarrhea (6).
Three additional questions:
- Can you postpone stools for 15 min? (always: 0, usually: 1, occasionally: 2, never: 3).
- Do you pass stools at night? (never: 0, occasionally: 1, usually: 2, always: 3).
- Do your bowel habits bother you? (no: 0, a little: 1, much: 2, very much: 3).
Gastrointestinal Quality of Life Index (GIQLI): Quality of Life (QoL) was measured using the validated GIQLI questionnaire with five subscales: physical role, large bowel function, emotional role, upper gastrointestinal tract function, and meteorism (7).
Data collection
First interview
In the first period [2012–2014] the patients were included postoperatively at the time of the interview. A control group operated with traditional right colectomy in the period 2007 to 2014 at Akershus University Hospital was extracted from the electronic patient journal (DIPS version 7.1). A single senior staff surgeon interviewed the patients by telephone regarding their bowel habits, including the Diarrhea Assessment Scale (DAS). On the same occasion, the patients were informed that they would receive a QoL-questionnaire. The only retrospective data gathered were data on stool frequency and urgency prior to the diagnosis of cancer. In the second period [2014–2018] consecutive patients (both patients belonging to the experimental group and controls) were included before the operation. Data regarding stool habits before the cancer diagnosis were collected at the time of the inclusion. They were informed that questionnaires concerning the quality of life and bowel habits would be sent to them at least 6 months after surgery.
Second interview
To see if the process of normalization seen at the first interview continues, we decided to repeat the interview for the patients who by the time of the first interview still had an abnormal bowel function. [Defined as patients fulfilling either of the following criteria in the first interview: (I) 4 or more stools per day (II) much or very much bothered by the bowel habits] (8,9).
The second interview was performed by telephone by the same senior staff surgeon as in the first interview. They were asked about their present bowel habits using DAS with the 3 additional questions (Extended DAS, 0–21, 0 is best). The GIQLI-questionnaire was not used in the second interview.
Statistical analysis
A statistician at Health Services Research Unit, Akershus University Hospital, analyzed the data (JCL). The impact of the D3 mesenterectomy versus the D2 mesenterectomy on DAS (with subscores) and GIQLI (with subscales) was analyzed using linear regression. The stool frequency score in the DAS was truncated at the lower and upper end but was used as if it has a one-to-one correspondence to frequency. This applies when we assume that few patients have less than one stool or more than four stools per day. To adjust for potential confounding in the groups the regression models were adjusted for gender, for age at the interview, and for the time elapsed from surgery to interview. The regression equations were in the form:
where Y represents either one DAS score, DAS-subscore, GIQLI score or one score on the GIQLI subscales. The D2 variable was coded as 1 for patients undergoing the D2 mesenterectomy and 0 for those undergoing the D3 mesenterectomy. All estimated differences should be read as the effect of receiving the D2 instead of D3 procedure. The analysis of stool frequency also contained a term for stool frequency before surgery.
Although answered on a 0 to 3 scale, the scores of the added questions were recoded into a dichotomous scale and analyzed with logistic regression, adjusting for gender, age and time between surgery and interview. Comparable regression analyses were modeled to examine the influence of previously identified anatomical variants on bowel function in the D3-group, and to compare the bowel function and Quality of Life separately for both genders.
Ethics committee approval
“Safe Radical D3 Right Hemicolectomy for Cancer through Preoperative Biphasic Multi-Detector Computed Tomography” is approved by the regional ethical committee (REK Sør-Øst no. 2010/3354), and registered at ClinicalTrials.gov on May 9th, 2011 (NCT01351714). Before inclusion, written informed consent from the patients was obtained. Comparison of bowel function after D3 to conventional (D2) right colectomy was also approved (REK Sør-Øst no. 2013/206 and 2015/403-3).
Results
First interview
Patients
- The experimental group: 83 patients (33 men, 37%) with a median age of 68 [27–78] were included in the study. The patients were split into two groups depending on whether they had one or more jejunal arteries above the MCA origin or not (for a complete overview of the distribution of jejunal arteries above MCA, see Table 1).
Table 1
Anatomical variations concerning jejunal arteries proximal to MCAGroup Arteries proximal to MCA (n) Incidence (n) Percent (%) a 0 16 19.3 b 1 22 26.5 2 27 32.5 3 11 13.3 4 4 4.8 5 1 1.2 >1 (number uncertain) 2 2.4 All patients – 83 100 - Patients without arteries above MCA: A total of 16 patients (9 men, 56%) with a median age of 70 [53–76];
- Patients with arteries above MCA: A total of 67 patients (25 men, 37.3%) with a median age of 67 [27–78] had one or more [1–5] arteries above MCA.
- The control group: a total of 61 patients (24 men, 39.3%) were included.
The groups (D2 and D3) were comparable for sex (P=0.85, Chi-square test of independence) and age (P=0.77, Mann-Whitney test). The time elapsed from surgery to interview was significantly longer in the D3-group (P<0.001, Mann-Whitney test).
The DAS and additional questions
All patients answered all DAS questions with additional questions (Extended DAS).
- D2 vs. D3: the results of the comparison between groups (D2 vs. D3) are presented in Tables 2,34) is no longer significant. The regression model reveals that the D2 group had 0.27 fewer stools per day than the D3 group (P=0.096). The only difference that is significant is a 0.351 lower consistency score (increased consistency) in the D2 group (P=0.012). The difference between the groups concerning DAS and its individual questions is even smaller when the patients without artery proximal to MCA are excluded (Table 3).
Table 2
Diarrhea Assessment Scale (DAS) with subscores and added questions. D2-group vs. D3-groupDAS questions (0–3) DAS Added questions (0–3) Stool frequency score Stool consistency score Stool urgency score Abdominal discomfort score Total score (0–12) Ability to postpone stool 15 m Need to defecate at night My bowel habits bother me D2, mean 0.46 0.64 0.64 0.49 2.07 0.81 0.26 0.65 D3, mean 1.00 0.94 0.94 0.33 3.01 1.17 0.20 0.43 Difference −0.54 −0.30 −0.30 0.16 −0.94 −0.36 −0.06 −0.22 Regression coefficientsa −0.273b −0.351 −0.209 0.141 −0.777 0.662cd 1.125c 0.617c 95% confidence interval −0.595, 0.049 −0.623, −0.079 −0.519, 0.102 −0.066, 0.348 −1.587, 0.033 0.231, 1.785 0.419, 2.911 0.274, 1.368 P value 0.096 0.012 0.186 0.181 0.060 0.424 0.810 0.237 DAS questions and scoring: Stool frequency: 0: 0–1 stool/day, 1: 2 stools/day, 2: 3 stools/day, 3: 4 or more stools/day; Bowel consistency: 0: all stools formed, 1: stools formed and loose, 2: stools loose, 3: watery stools; bowel urgency: 0: no urgency, 1: somewhat urgent, 2: urgent, 3: very urgent; abdominal discomfort: 0: no discomfort, 1: mild-moderate, 2: somewhat severe, 3: very severe discomfort. a, Adjusted for age, gender and time between surgery and interview; b, also adjusted for stool frequency before surgery; c, odds ratio estimated using logistic regression, adjusted for age, gender and time between surgery and interview; d, also adjusted for urgency before surgery.Table 3
Diarrhea Assessment Scale (DAS) with subscores and added questions. D2- patients vs. D3-patients with one or more jejunal arteries arising proximal to the middle colic arteryDAS questions (0–3) DAS Added questions (0–3) Stool frequency score Stool consistency score Stool urgency score Abdominal discomfort score Total score (0–12) Ability to postpone stool 15 m Need to defecate at night My bowel habits bother me D2, mean 0.46 0.50 0.64 0.49 2.07 0.88 0.20 0.43 D3a, mean 0.89 0.77 0.85 0.31 2.77 1.10 0.22 0.60 Difference −0.43 −0.27 −0.21 0.18 −0.70 −0.22 −0.08 −0.17 Regression coefficientsb −0.177c −0.276 −0.109 0.165 −0.524 0.695de 1.081d 0.823d 95% confidence interval −0.498, 0.144 −0.550, −0.003 −0.428, 0.210 −0.052, 0.383 −1.341, 0.293 0.233, 1.960 0.387, 2.936 0.349, 1.926 P value 0.277 0.048 0.500 0.135 0.207 0.499 0.879 0.654 DAS questions and scoring: Stool frequency: 0: 0–1 stool/day, 1: 2 stools/day, 2: 3 stools/day, 3: 4 or more stools/day; Bowel consistency: 0: all stools formed, 1: stools formed and loose, 2: stools loose, 3: watery stools; bowel urgency: 0: no urgency, 1: somewhat urgent, 2: urgent 3: very urgent; abdominal discomfort: 0: no discomfort, 1: mild-moderate, 2: somewhat severe, 3: very severe discomfort. a, Only D3 patients with one or more jejunal arteries arising proximal to the middle colic artery. b, Adjusted for age, gender and time between surgery and interview; c, also adjusted for stool frequency before surgery; d, odds ratio estimated using logistic regression, adjusted for age, gender and time between surgery and interview; e, also adjusted for urgency before surgery. - Vascular anatomy and bowel function after right colectomy with extended D3 mesenterectomy.
- The 3D reconstructions showed that 67 (76.1%) of the D3-patients had one or more JA [1–5] arising proximal to the root of the middle colic artery (cranial to the D3 area). The regression analysis revealed significantly lower scores for DAS, stool frequency, stool consistency, and stool urgency in patients with at least one artery proximal to the D3 area when compared to the patients without such an artery (see Table 4). A regression analysis with the exact number of arteries proximal to the dissection area as predictor produced regression coefficients (estimated change in response to an extra artery) for the following variables: DAS (−0.207, P=0.45, CI: −0.747, 0.334), Stools/day (−0.086, P=0.47, CI: −0.323, 0.151), GIQLI (0.510, P=0.822, CI: −4.00, 5.019).
Table 4
Diarrhea Assessment Scale (DAS) with subscores and added questions. D3-patients with vs. D3-patients without artery(ies) arising proximal to the middle colic arteryDAS questions (0–3) DAS Added questions (0–3) Stool frequency score Stool consistency score Stool urgency score Abdominal discomfort score Total score (0–12) Ability to postpone stool 15 m Need to defecate at night My bowel habits bother me D3 with artery, mean 0.89 0.77 0.85 0.40 2.77 1.10 0.22 0.60 D3 without art, mean 1.44 1.13 1.31 0.31 4.07 1.50 0.43 0.87 Difference −0.55 −0.36 −0.46 0.09 −1.30 −0.40 −0.21 −0.27 Regression coefficientsa −0.653b −0.437 −0.579 −0.095 −1.526 0.634cd 0.775c 0191c 95% confidence interval −1.120, −0.186 −0.851, −0.023 −0.996, −0.163 −0.384, 0.194 −0.346, −2.705 0.156, 2.795 0.189, 3.927 0.044, 0.671 P value 0.007 0.039 0.007 0.514 0.012 0.526 0.734 0.015 DAS questions and scoring: stool frequency: 0: 0–1 stool/day, 1: 2 stools/day, 2: 3 stools/day, 3: 4 or more stools/day; Bowel consistency: 0: all stools formed, 1: stools formed and loose, 2: stools loose, 3: watery stools; bowel urgency: 0: no urgency, 1: somewhat urgent, 2: urgent, 3: very urgent; abdominal discomfort: 0: no discomfort, 1: mild-moderate, 2: somewhat severe, 3: very severe discomfort. a, Adjusted for age, gender and time between surgery and interview; b, also adjusted for stool frequency before surgery; c, odds ratio estimated using logistic regression, adjusted for age, gender and time between surgery and interview; d, also adjusted for urgency before surgery. - Gender: when analyzing the genders separately, we found a non-significant difference of 0.396 stools per day (P=0.067) more in the female D3 group than in the female D2 group. Comparing the males separately, we found a small but significant difference in the consistency score which was 0.30 lower in the D2-group than in the D3-group (P=0.028). No other differences between the female or male groups were found concerning DAS, subscores or additional questions (see Tables 5,6
). Table 5
Diarrhea Assessment Scale (DAS) with subscores and added questions. D2-women vs. D3-womenDAS questions (0–3) DAS Added questions (0–3) Stool frequency score Stool consistency score Stool urgency score Abdominal discomfort score Total score (0–12) Ability to postpone stool 15 m Need to defecate at night My bowel habits bother me D2 women, mean 0.46 0.53 0.76 0.60 2.30 1.03 0.17 0.49 D3 women, mean 1.13 0.89 1.13 0.38 3.45 1.40 0.31 0.80 Differencea −0.67 −0.36 −0.37 0.22 −1.15 −0.37 −0.14 −0.31 95% confidence intervala −1.031, −0.300 −0.665, −0.066 −0.726, −0.011 −0.023, 0.473 −2.020, −0.236 −0.797, 0.061 −0.348, −0.056 −0.634, −0.015 P value (indep.T-test)a <0.01 0.017 0.044 0.065 0.014 0.092 0.177 0.061 Regression coefficientsb −0.396c −0.276 −0.179 0.147 −0.829 0.510de 0.511d 0.655d 95% confidence interval −0.820, 0.028 −0.646, 0.095 −0.604, 0.245 −0.146, 0.441 −1.916, 0.259 0.135, 2.003 0.126, 1.834 0.225, 1.895 P value (regression) 0.067 0.143 0.403 0.320 0.133 0.377 0.319 0.433 DAS questions and scoring: Stool frequency: 0: 0–1 stool/day, 1: 2 stools/day, 2: 3 stools/day, 3: 4 or more stools/day; Bowel consistency: 0: all stools formed, 1: stools formed and loose, 2: stools loose, 3: watery stools; bowel urgency: 0: no urgency, 1: somewhat urgent, 2: urgent, 3: very urgent; abdominal discomfort: 0: no discomfort, 1: mild-moderate, 2: somewhat severe, 3: very severe discomfort. a, Independent samples Test; b, adjusted for age, gender and time between surgery and interview; c, also adjusted for stool frequency before surgery; d, odds ratio estimated using logistic regression, adjusted for age, gender and time between surgery and interview; e, also adjusted for urgency before surgeryTable 6
Diarrhea Assessment Scale (DAS) with subscores and added questions. D2-men vs. D3-menDAS questions (0–3) DAS Added questions (0–3) Stool frequency score Stool consistency score Stool urgency score Abdominal discomfort score Total score (0–12) Ability to postpone stool 15 m Need to defecate at night My bowel habits bother me D2 men, mean 0.46 0.46 0.46 0.60 1.71 0.67 0.25 0.33 D3 men, mean 0.82 0.76 0.68 0.38 2.42 0.84 0.18 0.44 Difference −0.36 −0.30 −0.22 0.22 −0.71 −0.18 0.07 −0.11 95% confidence interval a −0.810, 0.080 −0.685, 0,72 −0.618, 0.182 −0.198, 0.335 −1.827, 0.395 −0.660, 0.297 −0.216, 0.353 −0.391, 0,175 P value (indep.T-test)a 0.106 0.110 0.279 0.608 0.202 0.454 0.633 0.448 Regression coefficients b −0.140c −0.487 −0.353 0.105 −0.911 1.182de 0.360d 1.878d 95% confidence interval −0.657, 0.377 −0.901, −0.054 −0.806, 0.100 −0.204, 0.415 −2.179, 0.356 0.243, 6.554 0.072, 1.709 0.530, 7.412 P value 0.589 0.028 0.124 0.499 0.155 0.838 0.195 0.342 DAS questions and scoring: Stool frequency: 0: 0–1 stool/day, 1: 2 stools/day, 2: 3 stools/day, 3: 4 or more stools/day; Bowel consistency: 0: all stools formed, 1: stools formed and loose, 2: stools loose, 3: watery stools; bowel urgency: 0: no urgency, 1: somewhat urgent, 2: urgent, 3: very urgent; abdominal discomfort: 0: no discomfort, 1: mild-moderate, 2: somewhat severe, 3: very severe discomfort. a, Independent samples Test; b, adjusted for age, gender and time between surgery and interview; c, also adjusted for stool frequency before surgery; d, odds ratio estimated using logistic regression, adjusted for age, gender and time between surgery and interview; e, also adjusted for urgency before surgery.- When we performed a T-test of the means for independent samples, without adjusting for age and for time from operation to interview, there was a significant difference between the two female groups concerning the DAS and the subscores, a result not found in males (see Tables 5,6
).
- When we performed a T-test of the means for independent samples, without adjusting for age and for time from operation to interview, there was a significant difference between the two female groups concerning the DAS and the subscores, a result not found in males (see Tables 5,6
Gastrointestinal Quality of Life Index (GIQLI)
One hundred and thirty-three (92.4%) patients answered the GIQLI forms. Four patients (3 men) in the D3 group and 7 (4 men) in the D2 group did not fill out the questionnaires. No significant differences were found between the D3 and D2 group concerning GIQLI or subscale scores (Table 7). A comparison between the D3 patients with and without arteries proximal to MCA revealed no differences except a non-significant difference in the subscore “Colon function” which was 2.15 lower in the group without artery (P=0.08, CI: −0.237, 4.537). This subscore contains questions regarding stool frequency and urgency.
Table 7
| Subscale division of GIQLI | Total GIQLI (0–144) | |||||
|---|---|---|---|---|---|---|
| Physical role (0–44) | Colon function (0–24) | Emotional role (0–32) | Upper GI function (0–32) | Meteorism (0–12) | ||
| D2, mean | 33.1 | 19.2 | 23.7 | 26.3 | 8.3 | 110,6 |
| D3, mean | 34.8 | 17.7 | 24.6 | 26.9 | 8 | 112,0 |
| Estimated difference (95% CI) | −0.301 (−3.220, 2.617) | 1.010 (−0.480, 2.499) | −0.059 (−2.126, 2.009) | −0.042 (−1.635, 1.551) | 0.339 (−0.510, 1.188) | 0.971 (−6.037, 7.978) |
| P value | 0.838 | 0.182 | 0.955 | 0.958 | 0.43 | 0.784 |
Second interview
In the second interview, only the patients presenting an abnormal bowel function (as defined above) at the first interview were included. Fourteen patients (2 men) fulfilled one or both criteria. Only 2 of them (both women) belonged to the D2 group. Six patients (1 man) fulfilled criteria A (4 or more stools/day). Ten patients (1 man) fulfilled criteria B (much or very much bothered by the bowel habits). Two of the patients (both of them without artery proximal to MCA) fulfilled both criteria.
Four patients (1 man) were excluded: 2 patients were dead (both D2), 2 patients received chemotherapy due to recurrence.
Mean observation time at 1. interview and 2. interview: 15.1 months and 56.4 months, respectively. Mean Extended DAS (0–21; 0=best) at the two interviews: 10.6 and 6.9, respectively. Eight out of 10 patients had lower Extended DAS score at the second interview. Seven of them did no longer fulfill our criteria for abnormal bowel function. (For further information see Table 8).
Table 8
| Patient | D | Prox. Art. (n) | Interview | Observation time (months) | Diarrhea Assessment Scale (DAS) | Added questions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (0–3) | Consistency (0–3) | Urgency (0–3) | Discomfort (0–3) | Postpone 15 m (0–3) | Stools at night (0–3) | Bowel habits bother (0–3) | Sum ext. DAS | |||||||
| Pat. 1 male | 3 | 0 | 1. | 11 | 3 | 3 | 2 | 0 | 2 | 3 | 1 | 14 | ||
| 2. | Excluded (Metachronous liver metastasis, operated and received chemotherapy. Not interviewed again) | |||||||||||||
| Pat. 2 female | 3 | 0 | 1. | 11 | 3 | 1 | 3 | 0 | 1 | 0 | 2 | 10 | ||
| 2. | 71 | 2 | 1 | 2 | 1 | 2 | 0 | 2 | 10 | |||||
| Pat. 3 female | 3 | 0 | 1. | 9 | 3 | 2 | 3 | 0 | 3 | 1 | 2 | 14 | ||
| 2. | 69 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Pat. 4 female | 3 | 2 | 1. | 23 | 3 | 2 | 1 | 0 | 1 | 1 | 1 | 9 | ||
| 2. | 83 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 4 | |||||
| Pat. 5 female | 3 | 2 | 1. | 13 | 3 | 2 | 2 | 1 | 3 | 0 | 3 | 14 | ||
| 2. | 25 | 3 | 1 | 3 | 0 | 3 | 0 | 3 | 13 | |||||
| Pat. 6 female | 3 | 2 | 1. | 15 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 7 | ||
| 2. | 20 | 2 | 1 | 2 | 0 | 2 | 0 | 2 | 9 | |||||
| Pat. 7 female | 3 | 2 | 1. | 6 | 2 | 2 | 1 | 0 | 0 | 0 | 3 | 8 | ||
| 2. | 68 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 4 | |||||
| Pat. 8 female | 3 | 2 | 1. | 10 | 2 | 2 | 3 | 1 | 2 | 1 | 2 | 13 | ||
| 2. | 34 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 5 | |||||
| Pat. 9 female | 3 | 3 | 1. | 23 | 3 | 1 | 1 | 1 | 1 | 0 | 0 | 7 | ||
| 2. | 84 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 6 | |||||
| Pat. 10 female | 2 | – | 1. | 25 | 2 | 3 | 3 | 2 | 3 | 0 | 3 | 16 | ||
| 2. | Excluded (Dead, cancer related) | |||||||||||||
| Pat. 11 female | 2 | - | 1. | 63 | 0 | 1 | 2 | 2 | 3 | 1 | 2 | 11 | ||
| 2. | Excluded (Dead, non-cancer related) | |||||||||||||
| Pat. 12 male | 3 | 1 | 1. | 28 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 13 | ||
| 2. | 33 | 2 | 2 | 1 | 1 | 2 | 0 | 0 | 8 | |||||
| Pat. 13 female | 3 | 3 | 1. | 13 | 0 | 1 | 1 | 1 | 1 | 2 | 2 | 8 | ||
| 2. | Excluded (Recurrence, receives chemotherapy. Not interviewed again) | |||||||||||||
| Pat. 14 female | 3 | 3 | 1. | 13 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 11 | ||
| 2. | 75 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | |||||
Discussion
The most important finding of this study is that the presence of jejunal arteries proximal to MCA have the potential to preserve the innervation despite extensive lymphadenectomy around the SMA distal to the MCA. The preoperative 3D-reconstructions of the SMA uncover a high vascular variability (2), especially concerning the branches irrigating the small bowel. The maps reveal a wide variety concerning the number and location of jejunal arteries proximal to the MCA. Most of the patients (in our study 81%) have at least one artery (in our study up to 5 arteries) above the MCA. Since the extrinsic nerves in the midgut are known to follow the arteries within the mesentery, jejunal branches leaving the SMA proximal to the area of dissection represent potential, undamaged pathways from the central SMP to the peripheral nerves following the arterial arcades, and also within the arcades (10). The fact that our study reveals a significantly better functional outcome after the denervation when at least one of these pathways is preserved, confirms the close relationship between arteries and extrinsic nerves in the midgut. Interestingly, the number of arteries proximal to the transection does not seem to matter as long as at least one bridging path remains. In the group without such an artery, both the total DAS-, the frequency-, and the consistency-scores are significantly higher than in the group with proximal arteries. Also, significantly more patients in the “without arteries proximal to MCA”-group said they were bothered by their stool habits (Tables 2-4).
Another observation we made, when comparing the groups without controlling for observation time and age, was that there was a significant gender-related difference. When we compared only men, and only women from the two groups (D2 vs. D3) separately by means of a T-test for independent samples, we found a significant difference between the women in the two groups, but not between the men. When we controlled for age and for the significantly longer observation time in the D2-group, this difference disappeared (Tables 5,6). This indicates that women are less able to compensate for the early postoperative bowel changes. Based on our clinical experience, we assume that this difference is related to the reduced defecation control which is often seen among elderly women (11). The clinical manifestation of this reduced control often appears when the consistency of the stool changes. Twelve out of the 14 patients in the group "abnormal bowel function" at the interview were women. Only two of them belonged to the D2-group. That is also an additional reason for adjusting the skewed distribution between the genders in our study (especially between the patients with and without arteries proximal to MCA).
Also, the time needed to regain the best possible postoperative bowel function appears to be longer than we first assumed. The second interview (Table 8) shows that the recovery of the bowel function continues over several months, even years. This also applies to the patients without arteries above MCA origin. Still, after the second year, the recovery seems to continue. If this expresses a more general adaptation or a nerve regeneration (12) is still not clear. In the regression model, we adjusted for the time from surgery to interview. One can dispute whether the time in the two groups is comparable since only the D3-patients are denervated. It is well possible that the normalization curves are not similar between the groups since one contains the component of denervation while the other does not. It might be that the recovery takes longer time in the D3-group, but it is difficult to draw any conclusions due to the significant longer observation time in the D2-group. At the interview, many of the patients in the D2-group had already recovered, so that it was not possible to determine if recovery occurred earlier than in D3-patients or not.
Limitations
As already demonstrated, the main limitation of this study is the significant difference in observation time between the D2- and the D3-group. The reason for the difference is the fact that D3-mesenterectomy became the standard procedure in our department, making it necessary to use controls from the time when the traditional D2 operation still was the surgery of choice. Another limitation is the lack of information about parity. Since women who have given birth to children are more prone to have a reduced anal function, it would have been interesting to know if the women who were most bothered by their bowel habits had a higher parity than the rest (11).
Conclusions
Our findings illustrate how standardized surgery may have different functional outcomes depending on individual anatomy. It also shows why it might be necessary to take the gender of the patient into account when informing the patients before the operation. Careful anamnesis concerning urgency and fecal incontinence can help us detect patients with an increased risk of annoying postoperative bowel habits.
Acknowledgments
Funding: Funding for the research and the publication came from Akershus University Hospital.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Bergamaschi and Mahir Gachabayov) for the series “Right Colon Cancer Surgery: Current State” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.07.04). The series “Right Colon Cancer Surgery: Current State” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study is approved by the regional ethical committee (REK Sør- Øst no. 2010/3354), and registered at ClinicalTrials.gov on May 9th, 2011 (NCT01351714). Before inclusion, written informed consent from the patients was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Today: International Agency for Cancer Research; 2018. Available online: http://gco.iarc.fr/today/online-analysis-table?v=2018&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=5&group_cancer=0&include_nmsc=1&include_nmsc_other=1
- Nesgaard JM, Stimec BV, Bakka AO, et al. Navigating the mesentery. A comparative pre- and per-operative visualization of the vascular anatomy. Colorectal Dis 2015;17:810-8. [Crossref] [PubMed]
- Thorsen Y, Stimec BV, Lindstrom JC, et al. Bowel Motility After Injury to the Superior Mesenteric Plexus During D3 Extended Mesenterectomy. J Surg Res 2019;239:115-24. [Crossref] [PubMed]
- Thorsen Y, Stimec B, Andersen SN, et al. Bowel function and quality of life after superior mesenteric nerve plexus transection in right colectomy with D3 extended mesenterectomy. Tech Coloproctol 2016;20:445-53. [Crossref] [PubMed]
- Ho YH, Low D, Goh HS. Bowel function survey after segmental colorectal resections. Dis Colon Rectum 1996;39:307-10. [Crossref] [PubMed]
- McMillan SC, Bartkowski-Doda L. Measuring bowel elimination. Instruments for Clinical Research in Health Care. Wilsonville, Oregon: Jones & Bartlett Inc. 1997.
- Sandblom G, Videhult P, Karlson BM, et al. Validation of Gastrointestinal Quality of Life Index in Swedish for assessing the impact of gallstones on health-related quality of life. Value Health 2009;12:181-4. [Crossref] [PubMed]
- Mitsuhashi S, Ballou S, Jiang ZG, et al. Characterizing Normal Bowel Frequency and Consistency in a Representative Sample of Adults in the United States (NHANES). Am J Gastroenterol 2018;113:115-23. [Crossref] [PubMed]
- Walter SA, Kjellstrom L, Nyhlin H, et al. Assessment of normal bowel habits in the general adult population: the Popcol study. Scand J Gastroenterol 2010;45:556-66. [Crossref] [PubMed]
- Sharov VA. Arkh Anat Gistol Embriol 1974;67:106-10. [Anatomy of the superior mesenteric plexus and of the nerves of the small intestine in man]. [PubMed]
- Boyle DJ, Knowles CH, Murphy J, et al. The effects of age and childbirth on anal sphincter function and morphology in 999 symptomatic female patients with colorectal dysfunction. Dis Colon Rectum 2012;55:286-93. [Crossref] [PubMed]
- Fatima J, Houghton SG, Sarr MG. Development of a simple model of extrinsic denervation of the small bowel in mouse. J Gastrointest Surg 2007;11:1052-6. [Crossref] [PubMed]
Cite this article as: Thorsen Y, Stimec BV, Lindstrom JC, Ignjatovic D. The effect of vascular anatomy and gender on bowel function after right colectomy with extended D3-mesenterectomy. Ann Laparosc Endosc Surg 2019;4:71.


