Radical surgery decreases overall morbidity and recurrence compared with conservative surgery for liver cystic echinococcosis: systematic review with meta-analysis
Introduction
Description of the disease
Hydatid disease or cystic echinococcosis (CE), caused by the tapeworm Echinococcus granulosus (E. granulosus), constitutes a serious public health problem in endemic areas (1). According to the World Health Organization (WHO), E. granulosus is endemic in areas of South America, Eastern Europe, Russia, Mediterranean countries, and China, where human incidence rates are 50 per 100,000 person-year (2). Grosso et al. (3), evaluating the worldwide epidemiology of liver hydatidosis, concluded that infection with Echinococcus granulosus remains a major public health issue in several countries, even in areas where it was previously at low levels.
The true prevalence of CE is difficult to evaluate owing to the high proportion of asymptomatic carriers (4). According to a Tunisian survey of 6,249 surgical interventions during the period 2001–2005, the surgical incidence rate per year ranged from 11 to 13.6 per 100,000 (5).
The liver is the predominant site of CE, encountered in 70% of patients (6). Surgery remains the cornerstone of liver cystic echinococcosis (LCE) management. Unroofing or pericystectomy techniques (7) are the most widely performed.
Why it is important to do this review
Currently, liver surgeons tend to prefer radical surgery (RS) such as pericystectomy or hepatic resection, which avoids recurrence from parasite vesiculation and decreases postoperative deep suppuration in the remnant cavity. On the other hand, general surgeons prefer conservative surgery (CS), an easier technique to perform by young general surgeons, associated with less intraoperative bleeding. Additionally, and despite the expertise of liver surgeons, RS remains difficult or impossible to perform when LCE is close to the hepatic veins or the inferior vena cava.
In 2004 a systematic review of the literature stated, “it is not possible to conclude which treatment, RS or CS, is better because the level of evidence was low” (8). One randomized trial, published in 2008 (9) including 32 patients, showed that RS had fewer cavity-related complications and early local recurrences than CS. The main critique of this trial is the small number of included patients. In 2015, the first published meta-analysis (10), included only five observational studies. Therefore, there is still limited evidence concerning the long- and short-term outcomes after surgery for LCE.
Aim of the study
This systematic review with meta-analysis aimed to investigate whether RS for LCE is superior to CS to decrease morbidity, mortality, and recurrence.
Methods
Search strategy and criteria for considering studies for this review
Electronic searches
An extensive electronic search of the relevant literature, published in English or French, was performed on April 13th, 2018 using the following databases: MEDLINE, Embase, the Cochrane Library, Scopus, INIST, Ovid, Science Direct, Google Scholar, Springer link, clinical key and the web of science. Keywords used for the final search in all databases were “liver” “hydatid cyst” “cystic echinococcosis” “surgery” “radical surgery” “conservative surgery” “pericystectomy” and “unroofing”.
Inclusion and exclusion criteria of studies
All relevant studies reporting a comparison between RS and CS to treat LCE, published in a peer-reviewed journal were considered for analysis. Data from non-comparative, editorials, letters to editors, review articles, and case series (fewer than ten cases) were excluded from the analysis.
Participants
Adults (age over 18 years) of either sex operated on for symptomatic but non-complicated LCE were included. We considered complicated LCE as (I) LCE ruptured into the biliary tract, (II) LCE involving the thorax, (III) LCE ruptured into the peritoneum.
Interventions
We studied two groups of surgical procedures: (I) RS, which included pericystectomy techniques (7), and hepatic resection; (II) CS, which corresponds to unroofing procedures, associated or not with other techniques, to manage the remnant cavity (7).
This meta-analysis was conducted according to the PRISMA 2009 checklist (11).
Outcome measures
Primary outcomes
The main outcome measure was overall postoperative morbidity. Overall postoperative morbidity was defined according to the Centers for Disease Control and Prevention classification (12): (I) extra surgical site (ESS) morbidity; (II) incisional surgical site (ISS) morbidity; (III) organ/space surgical site morbidity called “deep organ/space surgical site infection” which included the following: deep abdominal complications, deep abscess, cavity abscess, remnant cavity abscess, perihepatic collection, subhepatic abscess, and intra-abdominal abscess.
Secondary outcomes
- Postoperative mortality, defined as any and all deaths occurring during the hospital stay or within 30 days after discharge (13);
- Postoperative biliary leakage with or without biliary fistula: patients with bile draining through abdominal drains were classified as having biliary leakage. The biliary fistula was defined as drainage over 250 mL of bile daily for at least 3 days;
- Recurrence: recurrent hepatic hydatid disease was defined as the appearance of new active cysts after treatment of intrahepatic or extrahepatic disease (14).
Data collection and analysis
Selection of studies
Three authors (W Dougaz, I Samaali and M Khalfallah) independently retrieved full-texts of all studies that potentially met the inclusion criteria. If these three authors agreed that a study did not meet the eligibility criteria, other co-authors (W Dougaz, I Samaali, M Khalfallah and C Dziri) excluded it. If they disagreed, the conflict was resolved by discussion and consensus or by consulting two other members of the review team (C Dziri, A Fingerhut).
Extraction of data
Each author extracted the data independently from each study and performed the comparisons; all disparities were settled after a discussion with the senior authors (C Dziri, A Fingerhut).
Validity assessment of included studies
All studies that met the selection criteria were assessed for methodological quality by three authors (W Dougaz, I Samaali and M Khalfallah). The quality of the only randomized controlled trial (RCT) was assessed using the Jadad scoring system (15). Five was considered the best global score for RCTs. The Methodological Index for Non-Randomized Studies (MINORS) index was used to assess the quality of non-randomized trials (16). Twenty-four was considered the best global score for comparative studies.
Subgroup analysis and assessment of heterogeneity
Three subgroups were analyzed separately: (I) RCTs, (II) retrospective comparative studies using propensity-matching analysis and (III) retrospective comparative studies.
We used the Cochrane Chi2 test (Q-test) to assess heterogeneity. Significant heterogeneity was defined when the test showed a P<0.05. The I2 inconsistency test was used to estimate the degree of heterogeneity (17): an I2 between 0% and 50% was considered as probably not having substantial heterogeneity, whereas an I2, between 51% and 100% was considered as substantial heterogeneity.
Data synthesis and statistical analysis
Overall estimates of surgical procedure effect using odds ratios (OR) with their 95% confidence intervals (CIs) were calculated using the Mantel-Hansel method for random-effects. Results were presented in forest plots. All calculations were carried out using Review Manager 5.3 (Nordic Cochrane Center, Copenhagen, Denmark) freeware package.
Results
Retrieved reports
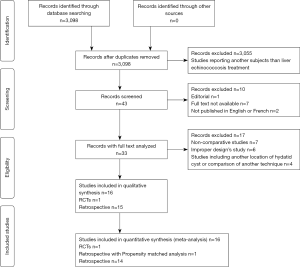
Overall, we identified 3,098 articles. After verification of inclusion and exclusion criteria, we retained 16 studies for final analysis: one RCT (9), one retrospective comparative study using propensity-matching analysis for comparison and 14 retrospective comparative studies (18-32) (PRISMA flow chart - Figure 1). Table 1 summarizes the results of the JADAD and MINORS score assessments.
Table 1
| Authors (reference) | Year of publication | Design | JADAD | MINORS | No. of patients R/C | Albendazole |
|---|---|---|---|---|---|---|
| Akbulut (18) | 2010 | Retrospective | – | 14/24 | 18/41 | Yes |
| Aydin (19) | 2008 | Retrospective | – | 13/24 | 92/129 | Yes |
| Birnbaum (20) | 2012 | Retrospective | – | 12/24 | 85/12 | No |
| Chautems (21) | 2003 | Retrospective | – | 12/24 | 57/21 | No |
| Cirenei (22) | 2001 | Retrospective | – | 14/24 | 164/134 | NR |
| El Malki (23) | 2014 | RPMA | – | 18/24 | 85/85 | Yes |
| Georgiou (24) | 2015 | Retrospective | – | 11/24 | 73/145 | Yes |
| Magistrelli (25) | 1991 | Retrospective | – | 14/24 | 64/71 | No |
| Mohkam (26) | 2014 | Retrospective | – | 16/24 | 52/27 | NR |
| Motie (27) | 2010 | Retrospective | – | 15/24 | 64/71 | Yes |
| Priego (28) | 2008 | Retrospective | – | 9/24 | 162/210 | No |
| Secchi (29) | 2009 | Retrospective | – | 13/24 | 396/748 | NR |
| Tagliacozzo (30) | 2011 | Retrospective | – | 14/24 | 240/214 | NR |
| Yüksel (9) | 2008 | RCT | 3/5 | – | 15/17 | Yes |
| Gupta (31) | 2011 | Retrospective | – | 14/24 | 61/33 | Yes |
| Yagci (32) | 2005 | Retrospective | – | 13/24 | 14/171 | Yes |
MINORS, Methodological Index of Non-Randomized Studies; JADAD, scale for assessing the quality of reports of randomized clinical trials; RPMA, retrospective with propensity-matched analysis; RCT, randomized controlled trial; NR, not reported.
Results of the search
Overall postoperative morbidity
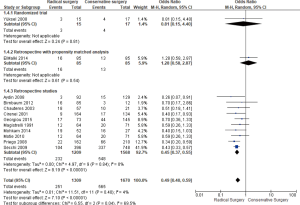
Twelve studies reported overall postoperative morbidity [251/1,309 (RS) and 565/1,670 (CS), respectively] (Figure 2); there was less overall postoperative morbidity in RS; (OR =0.49; 95% CI, 0.40–0.59, P=0.00001) with no substantial between-study heterogeneity (I2=4%, P=0.40).
Mortality
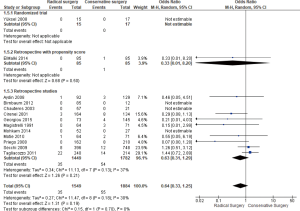
Thirteen studies contained data on mortality [35/1,549 (RS) and 54/1,884 (CS), respectively (P=0.91)]. There was no statistically significant difference found between the two groups RS and CS as shown in the forest plot (Figure 3).
Biliary leakage+/− fistula
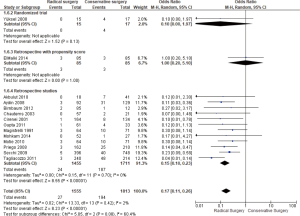
Twelve studies had exploitable data for biliary leakage+/− fistula (36/700 RS vs. 136/927 CS, respectively). As seen in Figure 4, there were fewer biliary leakage+/− fistula with RS (OR =0.35; 95% CI, 0.21–0.60, P=0.00001) than with CS with I2=24%.
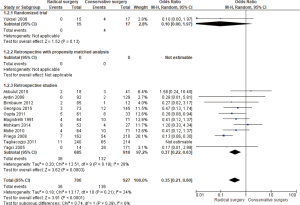
Recurrence
In the forest plot (Figure 5), of 14 studies [27/1,555 (RS) and 194/1,813 (CS), respectively, there was fewer recurrence found in RS; (OR =0.17; 95% CI, 0.11–0.26, P<0.00001).
Discussion
This meta-analysis showed that there were statistically significantly fewer overall morbidity, biliary leakage +/− fistula and recurrence in RS compared to CS. On the other hand, there was no statistically significant difference found concerning mortality between these two groups.
Meta-analyses were originally designed to include only data from RCTs (33). Shrier et al. (34) argued that systematic reviews and meta-analyses of interventional studies should include only RCTs because the RCTs are more valid for causal inference compared with the observational study design. However, as underlined by Cameron et al. (33), incorporating randomized studies with non-randomized studies in the same meta-analysis may be of interest in specific settings: (I) non-randomized studies can complement RCTs or address some of their limitations, such as short follow-up time, small sample size [as was the case concerning Yüksel et al.’s RCT (9) for this meta-analysis], highly selected population, high cost, and ethical restrictions; (II) simultaneous assessment of multiple treatments, including treatments that may not have been studied in RCTs is possible; (III) larger sample size and more diverse populations may improve the generalizability of the findings (33).
Moreover, while these two types of designs have their strengths and weaknesses (34), a review of empirical studies suggests that meta-analyses based on observational studies can produce estimates of effect similar to those from meta-analyses based on RCTs only (34). In addition, Hannan et al. (35) stated that the design and ultimate conduct of the study is the principal criterion to consider, not the type of study. However, it must be emphasized that causality cannot be inferred with the same strength as if all studies were randomized.
Agreements and disagreements with other studies or reviews
The meta-analysis, published in 2015 (10), including five observational studies, concluded that RS, especially total pericystectomy, had fewer complications, lower postoperative recurrence, and a lower incidence of biliary fistula and infection, as compared with CS. However, there are several statistical flaws in this meta-analysis as some relevant articles were lacking (20-26,28,30). Furthermore: (I) for “biliary leakage and fistula”, the authors did not extract data exactly as mentioned in the articles of Akbulut et al. (18), Aydin et al. (19) and Motie et al. (27); (II) for “mortality” and “overall morbidity”, Akbulut et al.’s article (18) was not concerned by these two criteria and the authors reported that the mortality and overall morbidity were nil; (III) for “length of hospital stay”, the forest plot showed no statistical difference with a high heterogeneity and the authors did not explain this heterogeneity. In addition, as concerns, the outcome “postoperative biliary leakage”, their conclusion (10) was a lower incidence of biliary fistula and infection in favor of RS but in the text, it was mentioned that there was no statistical significance found between the two groups.
A recent systematic review with meta-analysis in “Frontiers in medicine” (36), including 19 studies, concluded that RS may reduce the risk of postoperative complications and recurrence. Heterogeneity between included studies was considerable in this review and the authors were forced to withdraw several studies to solve this problem. Among studies included in this review, two did not meet the selection criteria that we consider in our meta-analysis (37,38).
Potential weaknesses in our study
Our meta-analysis included one RCT, one observational study with propensity matching and 14 retrospective comparative studies. Cameron et al. (33) also emphasized that “Including low-quality, non-randomized comparative cohort studies, could perpetuate the biases that are unknown, unmeasured, or uncontrolled.”
Even if the sample size of the only RCT included herein was 32, 0.51% of the total, we cannot eliminate unknown confounders that might have skewed the results of mixing randomized with observational propensity-matched or retrospective unmatched comparative studies in our analysis, therefore no causality can be inferred. Moreover, three potentially relevant articles were not available with full texts and therefore were not analyzed: one in Chinese (39) and two in English (40,41).
In conclusion, in the absence of multicenter RCTs, this comprehensive meta-analysis of the available evidence suggests that RS is superior to CS in treating hydatid disease of the liver.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.08.01). AF served as the unpaid Guest Editor of the series and serves as the Editor-in-Chief of Annals of Laparoscopic and Endoscopic Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dziri C. Hydatid disease-continuing serious public health problem: introduction. World J Surg 2001;25:1-3. [Crossref] [PubMed]
- Brunetti E, Kern P, Vuitton DA, et al. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010;114:1-16. [Crossref] [PubMed]
- Grosso G, Gruttadauria S, Biondi A, et al. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J Gastroenterol 2012;18:1425-37. [Crossref] [PubMed]
- Rossi P, Tamarozzi F, Galati F, et al. The first meeting of the European Register of Cystic Echinococcosis (ERCE). Parasit Vectors 2016;9:243. [Crossref] [PubMed]
- Chahed MK, Bellali H, Touinsi H, et al. Distribution of surgical hydatidosis in Tunisia, results of 2001-2005 study and trends between 1977 and 2005. Arch Inst Pasteur Tunis 2010;87:43-52. [PubMed]
- Sayek I, Yalin R, Sanac Y. Surgical treatment of hydatid disease of the liver. Arch Surg 1980;115:847-50. [Crossref] [PubMed]
- Dziri C, Nouira R. Surgical treatment of liver hydatid disease by laparotomy. J Visc Surg 2011;148:e103-10. [Crossref] [PubMed]
- Dziri C, Haouet K, Fingerhut A. Treatment of hydatid cyst of the liver: where is the evidence? World J Surg 2004;28:731-6. [Crossref] [PubMed]
- Yüksel O, Akyürek N, Şahin T, et al. Efficacy of Radical Surgery in Preventing Early Local Recurrence and Cavity-Related Complications in Hydatic Liver Disease. J Gastrointest Surg 2008;12:483-9. [Crossref] [PubMed]
- He YB, Yao G, Tuxun T, et al. Efficacy of radical and conservative surgery for hepatic cystic echinococcosis: a meta-analysis. Int J Clin Exp Med 2015;8:7039-48. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions ofsurgical wound infections. Am J Infect Control 1992;20:271-4. [Crossref] [PubMed]
- Evans M, Pollock AV. Trials on trials: a review of trials of antibiotic prophylaxis. Arch Surg 1984;119:109-13. [Crossref] [PubMed]
- Sielaff TD, Taylor B, Langer B. Recurrence of hydatid disease. World J Surg 2001;25:83-6. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712-6. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Akbulut S, Senol A, Sezgin A, et al. Radical vs conservative surgery for hydatid liver cysts: experience from single center. World J Gastroenterol 2010;16:953-9. [Crossref] [PubMed]
- Aydin U, Yazici P, Onen Z, et al. The optimal treatment of hydatid cyst of the liver: radical surgery with a significant reduced risk of recurrence. Turk J Gastroenterol 2008;19:33-9. [PubMed]
- Birnbaum DJ, Hardwigsen J, Barbier L, et al. Is hepatic resection the best treatment for hydatid cyst? J Gastrointest Surg 2012;16:2086-93. [Crossref] [PubMed]
- Chautems R, Bubler L, Gold B, et al. Long-term results after complete or incomplete surgical resection of liver hydatid disease. Swiss Med Wkly 2003;133:258-62. [PubMed]
- Cirenei A, Bertoldi I. Evolution of surgery for liver hydatidosis from 1950 to Today: Analysis of a personnel experience. World J Surg 2001;25:87-92. [Crossref] [PubMed]
- El Malki HO, Souadka A, Benkabbou A, et al. Radical versus conservative surgical treatment of liver hydatid cysts. Br J Surg 2014;101:669-75. [Crossref] [PubMed]
- Georgiou GK, Lianos GD, Lazaros A, et al. Surgical management of hydatid liver disease. Int J Surg 2015;20:118-22. [Crossref] [PubMed]
- Magistrelli P, Masetti R, Coppola R, et al. Surgical treatment of hydatid disease of the liver. Arch Surg 1991;126:518-22. [Crossref] [PubMed]
- Mohkam K, Belkhir L, Wallon M, et al. Surgical management of liver hydatid disease: subadventitial cystectomyversus resection of the protruding dome. World J Surg 2014;38:2113-21. [Crossref] [PubMed]
- Motie MR, Ghaemi M, Aliakbarian M, et al. Study of the Radical vs. Conservative Surgical Treatment of the Hepatic Hydatid Cyst: A 10-Year Experience. Indian J Surg 2010;72:448-52. [Crossref] [PubMed]
- Priego P, Nuño J, López Hervás P, et al. Hepatic hydatidosis. Radical vs. conservative surgery: 22 years of experience. Rev Esp Enferm Dig 2008;100:82-5. [Crossref] [PubMed]
- Secchi MA, Pettinari R, Mercapide C, et al. Surgical management of liver hydatidosis: a multicentre series of 1412 patients. Liver Int 2010;30:85-93. [Crossref] [PubMed]
- Tagliacozzo S, Miccini M, Amore Bonapasta S, et al. Surgical treatment of hydatid disease of the liver: 25 years of experience. Am J Surg 2011;201:797-804. [Crossref] [PubMed]
- Gupta N, Javed A, Puri S, et al. Hepatic hydatid: PAIR, drain or resect? J Gastrointest Surg 2011;15:1829-36. [Crossref] [PubMed]
- Yagci G, Ustunsoz B, Kaymakcioglu N, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg 2005;29:1670-9. [Crossref] [PubMed]
- Cameron C, Fireman B, Hutton B, et al. Network meta-analysis incorporating randomized controlled trials and non-randomized comparative cohort studies for assessing the safety and effectiveness of medical treatments: challenges and opportunities. Syst Rev 2015;4:147. [Crossref] [PubMed]
- Shrier I, Boivin JF, Steele RJ, et al. Should Meta-Analyses of Interventions Include Observational Studies in Addition to Randomized Controlled Trials? A Critical Examination of Underlying Principles. Am J Epidemiol 2007;166:1203-9. [Crossref] [PubMed]
- Hannan EL. Randomized Clinical Trials and Observational Studies Guidelines for Assessing Respective Strengths and Limitations. JACC Cardiovasc Interv 2008;1:211-7. [Crossref] [PubMed]
- Pang Q, Jin H, Man Z, et al. Radical versus conservative surgical treatment of liver hydatid cyst: a meta-analysis. Front Med 2018;12:350-9. [Crossref] [PubMed]
- Gollackner B, Längle F, Auer H, et al. Radical surgical therapy of abdominal cystic hydatid disease: factors of recurrence. World J Surg 2000;24:717-21. [Crossref] [PubMed]
- Yorganci K, Sayek I. Surgical treatment of hydatid cysts of the liver in the era of percutaneous treatment. Am J Surg 2002;184:63-9. [Crossref] [PubMed]
- Wen H, Shao YM, Zhao JM, et al. Clinical analysis of surgical treatment for human hepatic cystic and alveolar echinococcosis. Chinese J Dig Surg 2007;6:3-18.
- Abbas M, Nafeh AI, Youssef YF, et al. Conservative versus radical surgery for treatment of uncomplicated hepatic hydatid cysts. J Egypt Soc Parasitol 2006;36:559-76. [PubMed]
- Elsebaie SB, El-Sebae MM, Esmat ME, et al. Modified endocystectomy versus pericystectomy in echinococcus granulosus liver cysts: a randomized controlled study, and the role of specific anti-hydatid IgG4 in detection of early recurrence. J Egypt Soc Parasitol 2006;36:993-1006. [PubMed]
Cite this article as: Dziri C, Dougaz W, Samaali I, Khalfallah M, Jerraya M, Mzabi R, Bouasker I, Nouira R, Fingerhut A. Radical surgery decreases overall morbidity and recurrence compared with conservative surgery for liver cystic echinococcosis: systematic review with meta-analysis. Ann Laparosc Endosc Surg 2019;4:92.