What does robotic right colectomy add to its laparoscopic counterpart?
Introduction
The right colectomy, which is applied in the treatment of the malign lesions that develop in the segment between the colonic hepatic flexure and the ileocecal valve, can be performed with either open, laparoscopic or robotic method.
Worldwide, there are ongoing efforts to develop minimally invasive techniques in colorectal surgery without compromising the oncological surgical principles. In terms of minimally invasive colorectal surgery, the most common technique is laparoscopic approach. Yet, robotic surgery has come to the forefront in the recent years as a technique with frequent use.
In the previous studies investigating colon surgery, it was reported that laparoscopic surgery (LS) is superior to open surgery regarding blood loss, hospital stay, time to bowel function, wound complications and postoperative pain (1-4). However, concerns have been raised toward the oncologic safety of the laparoscopic technique in rectal cancer surgery, and the repercussions these findings have been reflected to right-sided tumors as well. The reason why LS has not become the gold standard surgery, even though oncological results are accepted in right colon cancers, may be due to the technical difficulties and variable vascular anatomy (5,6). To overcome these challenges, the conventional LS was tailored to develop hand-assisted laparoscopy technique but the results did not mirror the plausible effects of the minimally invasive surgery. Thus, the search for a gold standard minimally-invasive technique has been continued to augment the surgical outcomes of the LS technique (7-10).
With the advantages of robotic surgery (RS) like stable camera platform, three-dimensional imaging, improved ergonomics, tremor elimination, ambidextrous capability and motion scaling, an important step may be taken in minimally-invasive surgery to eliminate the concerns of the laparoscopy. The advantages of the robotic surgery—especially on the right colectomy frontier, are more likely to be pronounced with the implementation of complete mesocolic excision (CME) technique, which follows the same principles of total mesorectal excision. Furthermore, robotic surgery may ease and promote intracorporeal anastomosis which is challenging to perform with the laparoscopic approach
In this paper, we discussed the contributions of robotic right colectomy in the treatment of right colon cancers in light of the most recent literature.
Technical aspects
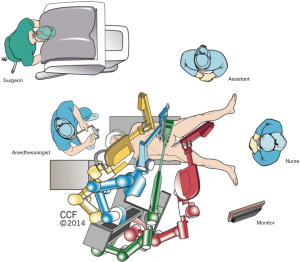
Operation room is designed as shown in Figure 1. The patient is positioned to modified lithotomy. Four or five robotic ports, one assistant port and one Air Seal (Conmed, Largo, FL, USA) port are preferred for the procedure. Initially a 12-mm incision is made left to the umbilicus to be used as the camera site. After placement of the camera other ports are placed diagonally running from 4–5 cm above the pubis in the midline toward left upper abdomen as shown in Figure 2. Additionally, an assistant port is placed in the left lateral abdomen.
The dissection is performed in a medial to lateral fashion. Central vascular dissection with lymphadenectomy at the origin of the ileocolic, right colic, and right branches of middle colic vessels and the loop of Henle is performed along the left border of the superior mesenteric vein. The colon is then dissected from the fascia of Gerota and the retroperitoneum, preserving the duodenum, ureters, and gonadal vessels. Take down of the hepatic flexure is then performed. Subsequently, indocyanine green dye is used to evaluate colonic perfusion and determine the transection point. An intracorporeal, side-to-side, isoperistaltic anastomosis is formed after the transection of the colon and ileum. A Pfannenstiel incision is made for the specimen extraction.
Laparoscopic versus robotic right colectomy
Short term outcomes
Harvested lymph node
In colorectal carcinoma, the status of the lymph nodes during surgery is one of the most important criteria for prognosis. Studies conducted so far showed that there are no statistically significant differences between RS and LS in terms of the number of the lymph nodes removed (11-14). D’Annibale et al. (15) shared their experiences in a review, and reported that the mean number of harvested lymph nodes was 18.7±7.2 in patients who had undergone robotic right colectomy. They claimed that the robotic technique could provide a better standardization, and it would especially bring high benefits in a great majority of patients in terms of ensuring accurate lymphatic resection (15). In some other studies; however, more harvested lymph nodes were reported in RS at a statistically significant level (16,17). Solaini et al. (18) conducted a meta-analysis, and reported that the robotic approach could result in more harvested lymph nodes for right hemicolectomy (P=0.057). However, the data on lymph node harvest are granular as oncologic principles have changed significantly and a significant fraction of the laparoscopic studies does not include high ligation of the vessels. Nevertheless, robotic surgery brings news approaches to lymph node dissection, which may enhance the scope of identifying nodes in the mesentery (19).
Conversion to open surgery
It was emphasized that the rates of conversion to open surgery were lower in RS groups in right colectomies (16,18).
In a recent paper which nearly spanned over (20) 300,000 right colectomies stated that the conversion to open surgery caused higher mortality, higher overall morbidity, longer length of hospitalization, and increased hospital charges.
Ma et al. (21) conducted a meta-analysis, and determined that the rate of conversion to open surgery in the RS group were lower [odds ratio (OR) =0.34, 95% Cl: 0.15–0.75; P=0.008).
Megevan et al. (12) conducted a study and emphasized that the management of complications that could develop intraoperatively was much easier, and for this reason, the conversion rates to open surgery were lower. In this study, the conversion to open surgery rate was 14% (7/50) in LS group compared to 0% (0/50) in the RS group (P<0.001, adjusted P=0.001).
Duraes et al. (22) reported that there were no differences between laparoscopy and open surgery in terms of 5-year OS (P=0.258), DFS (P=0.070), cancer-specific survival (CSS) (P=0.207), or recurrence (P=0.216). On the other hand, conversion to open surgery had a worse OS (P=0.010) and DFS (P=0.006) when compared to laparoscopic only.
The low rate of conversion to open surgery might indirectly lead to the low mortality and morbidity rates in robotic surgery.
Blood loss
Ma et al. (21) conducted a meta-analysis, and reported that the intraoperative bleeding was lower in RS group compared to LS group at a statistically significant level. Trastulli et al. (16) also reported that there was lower intraoperative blood loss in patients who underwent RS. Park et al. (13) conducted a randomized study for right colectomies and detected no significant difference between the groups in terms of estimated blood loss. As a conclusion, it is understood that LS has no superiority in terms of estimated blood loss. RS has the potential to decrease intraoperative blood loss but this has not been supported by randomized trials yet.
Type of anastomosis
In previous studies, it was emphasized that IA might be carried out safely in RS as it is the case in LS. Because of non-ergonomic tools, EA is preferred sometimes in LS (23). In a randomized study that was conducted by Park et al. (13), it was observed that more EA was preferred in LS group at a significant level.
It is possible to perform a smaller abdominal incision for specimen extraction in more convenient sites with intracorporeal anastomosis (IA). Better cosmetic results, lower morbidity, decreased wound complications and incisional hernia rates and less postoperative pain are the main advantages of IA (14,24-28).
Feroci et al. (29) conducted a meta-analysis comparing IA versus EA after laparoscopic right colectomy for cancer. They emphasized that IA results in shorter time to bowel functions and first flatus, earlier oral intake, decreased necessity of analgesic usage and length of hospital stay.
Solaini et al. (17) conducted a study and compared the robotic and laparoscopic right colectomies in which anastomoses were done in an intracorporal manner. They compared the sub-groups who underwent surgeries for malignant lesions and reported that the harvested lymph node count was more in RS, time to first flatus was shorter, re-admission to hospital was less; however, operation durations was longer at statistically significant levels.
Based on these data, it may be claimed that robotic surgery allows more IA, and therefore, lower morbidity rates might be achieved.
Either isoperistaltic or antiperistaltic ileocolic anastomosis can be carried out following right hemicolectomy. The influence of peristaltism on the anastomosis could not been revealed, yet. Recently, in a double-blind, randomized, prospective trial; it has been stated that isoperistaltic and antiperistaltic anastomosis are comparable in terms of safety and functionality (30). We prefer an intracorporeal, isoperistaltic, side-to-side ileocolic anastomosis.
Anastomotic bleeding and leakage
In previous studies, no significant differences were detected between the RS and LS in right colon cancer in terms of anastomotic bleeding and leakage (12,17-21,31).
Operative time
Megevand et al. (12) conducted a study for right colectomy and determined that the median total operative time was 160 minutes (IQR: 180–230) in the LS group, and 204 minutes in the RS group (IQR: 180–230), which was statistically significant. In a meta-analysis conducted by Ma et al. (21), it was emphasized that operation time was longer in the RS group. However, no significant differences were detected between the groups in terms of operation duration in 3 other studies which did not include undocking duration (14,32,33). In addition, it was also reported that as the number of right colectomies increase, operation time could decrease in time. As the surgical team gains experience and reaches the end of the learning curve for the robotic right colectomy, one might expect to see shorter operation time (15,34,35).
Solaini et al. (18) conducted a meta-analysis study and compared robotic versus laparoscopic right colectomies, and reported that there was no significant difference in operative time in a sub-group of patients who had had EA. Patients who received IA could not be compared in this study due to insufficient data. On the other hand, in a study in which only IA was carried out, it was found that operative time was higher in the RS group, as expected (17).
Limited tactile feedback, depending on a skilled bedside assistant for counter-traction and placement of various instruments and the separation of surgeon and operating bed may cause delay. Studies in the literature show that usually RS takes longer time than LS (16,17,36-40)
Kang et al. (14), on the other hand, reported that there were no differences between RS and LS in terms of operative time. Since learning curve plays an important role on operative time, future studies should be designed to include a more homogenous sample of surgeons in terms of robotic experience.
Quality of the resected specimen
CME was firstly described by Hohenberger et al. (41). They showed that patients undergoing CME had a lower local recurrence rate (3.6%) and 5-year cancer related survival rate was increased (89.1%) in this patient group compared to non-CME group. Sharp dissection along the mesocolic plane with true central vascular ligation at main arteries’ and veins’ roots is the key point of this technique (41-43). Ozben et al. (44) conducted a study and reported the results of 37 patients who underwent robotic surgery with the CME technique. It was emphasized that oncologic results were better and a better-quality specimen might be achieved with robotic CME. Spinoglio et al. (45) compared robotic versus laparoscopic right colectomy with CME for the treatment of colon cancer and stated that both approaches were safe and feasible. They emphasized that RS is more advantageous in terms of IA and it also eases the dissection to reach the origin of the vessels. However, there is a need for higher evidence studies as it is hard to draw conclusions since most of the laparoscopic studies inherently included the era before the invention of the complete mesocolic excision. Also, surgical community lacks a standardized method when it comes to evaluate the quality of the specimen in right hemicolectomies, unlike total mesorectal excision. All these factors could lead to a subjective evidence in the literature in terms of specimen quality.
Time to return of bowel function
It was thought that ergonomic instruments create less trauma and less traction on mesocolon in RS, thus bowel functions start earlier (12). Ma et al. (21) conducted a meta-analysis in which 4 studies were included, and reported that there was no significant difference between the groups. In another study, interval to bowel movement was reduced in RS (P<0.001).
Time to first flatus
Recent studies reported that the median time to first flatus was reduced in the RS group. (12,15-18,23,46-48). Solaini et al. (18) emphasized that no significant difference was observed in terms of time to first flatus in patients who received extracorporeal anastomosis in RS and LS groups.
Hospital stay
Length of stay was lower in RS group than LS. The higher number of complications in LS group can partially explain the longer median length of stay (12). However, in another study in which IA was carried out in all patients, it was reported that there was no difference between the groups in terms of hospital stay (17).
Long term outcomes
No adequate prospective randomized studies have been reported in the literature yet for the long-term results of robotic right colectomy. In a randomized study which included 75 patients (13), the long-term oncologic results of Robotic (n=35) versus Laparoscopic (n=35) right colectomy were discussed. No significant difference was seen in 3-year and 5-year disease-free survival (DFS). Three-year overall survival (OS) rate did not differ significantly as well. Stage-specific analysis showed no difference in DFS and OS between treatment groups. They confirmed that LS has no superiority in terms oncological clearance and 3-year survival rate.
D’Annibale et al. (15) conducted a study including 50 patients who underwent robotic right colectomy and reported the survival rates as following: DFS [90% (45/50)] and OS [92% (46/50)] without any statistical difference.
In another study Kang et al. (14) included patients who had right-sided colon cancer and evaluated the survival rates in patients who underwent open, laparoscopic and robotic right colectomy. Five-year DFS did not differ between the groups (87.7%, 84%, and 89.5%, RS and LS respectively).
Cost
It may be claimed that the most important disadvantage of robotic surgery is its cost. Apart from the capital cost of purchasing the robotic device, the lack of re-payments for robotic surgery constitutes a major problem (35). However, it is necessary to perform head-to-head comparisons including the postoperative period, since RS may provide lower morbidity rates compared to LS, hence the overall costs might be similar to those of LS. Also, it is expected that cost-effectivity might increase in the future if a more competitive industrial environment is created along with more surgeons utilizing the robotic platform more efficiently.
Learning curve
Parisi et al. (49) conducted a study and reported that the surgeons with a laparoscopic acumen could obtain good oncologic results from the beginning for robotic right colectomy. It was shown that surgeons would need to perform 44 robotic right colectomies to optimize their operative time, conversion rate and harvested lymph node number.
Cosmetic
Selecting incisions with which better results may be achieved in cosmetic terms is an important advantage in RS because of lower rate of conversion to open surgery and higher chance of intracorporeal anastomosis.
Conclusions
Laparoscopic right colectomy, which has acceptable oncologic results in right colon cancer surgery, is a minimally-invasive surgical procedure that has been globally performed but has many rooms for improvement. We believe that RS has the potential to further enhance the capabilities of the minimally invasive surgery, and right colon surgery would be a great frontier to achieve better perioperative results. The merits of the robotic platform may aid to perform CME with its endo-wristed instruments and an IA, which could be challenging to perform with a laparoscopic approach. Though longer operative time and higher costs appear to be the main obstacles in RS, the competition in the market and new generation surgeons with RS training may mitigate these factors:
- Long-term oncologic results are similar to those of the LS. However, a better-quality specimen resection might be carried out with robotic CME;
- The conversion to open surgery rates and estimated blood loss may be lower in robotic surgery;
- Due to instrumental facilities in robotic surgery, intracorporeal anastomosis can be attempted more often than LS. The ability to remove the specimen through Pfannenstiel incision may reduce incisional hernia rates and postoperative pain;
- There are indirect contributions like low rates of conversion to open surgery and high intracorporeal anastomosis rates, lower morbidity, shorter time to bowel function, achieving better results in cosmetic terms;
- We believe that although it seems that surgical procedure has higher costs, the costs must be calculated by considering the perioperative complications and hospital stay. Lower costs can be achieved in the future with a more competitive industrial environment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Bergamaschi and Mahir Gachabayov) for the series “Right Colon Cancer Surgery: Current State” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.10.04). The series “Right Colon Cancer Surgery: Current State” was commissioned by the editorial office without any funding or sponsorship. EG serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jun 2019 to May 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jayne DG, Thorpe HC, Copeland J, et al. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 2010;97:1638-45. [Crossref] [PubMed]
- Weeks JC, Nelson H, Gelber SClinical Outcomes of Surgical Therapy (COST) Study Group, et al. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA 2002;287:321-8. [Crossref] [PubMed]
- Lacy AM, Delgado S, Castells A, et al. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg 2008;248:1-7. [Crossref] [PubMed]
- Guillou PJ, Quirke P, Thorpe H, et al. MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718-26. [Crossref] [PubMed]
- Bailey MB, Davenport DL, Vargas HD, et al. Longer operative time: deterioration of clinical outcomes of laparoscopic colectomy versus open colectomy. Dis Colon Rectum 2014;57:616-22. [Crossref] [PubMed]
- Künzli BM, Friess H, Shrikhande SV. Is laparoscopic colorectal cancer surgery equal to open surgery? An evidence based perspective. World J Gastrointest Surg 2010;2:101-8. [Crossref] [PubMed]
- Ding J, Xia Y, Liao GQ, et al. Hand-assisted laparoscopic surgery versus open surgery for colorectal disease: a systematic review and meta-analysis. Am J Surg 2014;207:109-19. [Crossref] [PubMed]
- Tajima T, Mukai M, Yamazaki M, et al. Comparison of handassisted laparoscopic surgery and conventional laparotomy for colorectal cancer: Interim results from a single institution. Oncol Lett 2014;8:627-32. [Crossref] [PubMed]
- Kanemitsu Y, Komori K, Kimura K, et al. D3 Lymph Node Dissection in Right Hemicolectomy with a No-touch Isolation Technique in Patients With Colon Cancer. Dis Colon Rectum 2013;56:815-24. [Crossref] [PubMed]
- Kornmann VN, Hagendoorn J, van Koeverden S, et al. Totally laparoscopic right hemicolectomy with intracorporeal anastomosis is a technically and oncologically safe procedure. Acta Chir Belg 2013;113:439-43. [Crossref] [PubMed]
- Guerrieri M, Campagnacci R, Sperti P, et al. Totally robotic vs 3D laparoscopic colectomy: A single centers preliminary experience. World J Gastroenterol 2015;21:13152-9. [Crossref] [PubMed]
- Mégevand JL, Amboldi M, Lillo E, et al. Right colectomy: consecutive 100 patients treated with laparoscopic and robotic technique for malignancy. Cumulative experience in a single centre. Updates Surg 2019;71:151-6. [Crossref] [PubMed]
- Park JS, Kang H, Park SY, et al. Long-term oncologic after robotic versus laparoscopic right colectomy: a prospective randomized study. Surg Endosc 2019;33:2975-81. [Crossref] [PubMed]
- Kang J, Park YA, Baik SH, et al. A Comparison of Open, Laparoscopic, and Robotic Surgery in the Treatment of Right-sided Colon Cancer. Surg Laparosc Endosc Percutan Tech 2016;26:497-502. [Crossref] [PubMed]
- D'Annibale A, Pernazza G, Morpurgo E, et al. Robotic right colon resection: evaluation of first 50 consecutive cases for malignant disease. Ann Surg Oncol 2010;17:2856-62. [Crossref] [PubMed]
- Trastulli S, Coratti A, Guarino S, et al. Robotic right colectomy with intracorporeal anastomosis compared with laparoscopic right colectomy with extracorporeal and intracorporeal anastomosis: a retrospective multicentre study. Surg Endosc 2015;29:1512-21. [Crossref] [PubMed]
- Solaini L, Cavaliere D, Pecchini F, et al. Robotic versus laparoscopic right colectomy with intracorporeal anastomosis: a multicenter comparative analysis on short-term outcomes. Surg Endosc 2019;33:1898-902. [Crossref] [PubMed]
- Solaini L, Bazzocchi F, Cavaliere D, et al. Robotic versus laparoscopic right colectomy: an updated systematic review and meta-analysis. Surg Endosc 2018;32:1104-10. [Crossref] [PubMed]
- Ozben V, Cengiz TB, Bayraktar O, et al. Identification of mesenteric lymph nodes in robotic complete mesocolic excision by near-infrared fluorescence imaging. Tech Coloproctol 2016;20:195-6. [Crossref] [PubMed]
- Moghadamyeghaneh Z, Masoomi H, Mills SD, et al. Outcomes of Conversion of Laparoscopic Colorectal Surgery to Open Surgery. JSLS 2014; [Crossref] [PubMed]
- Ma S, Chen Y, Chen Y, et al. Short-term outcomes of robotic-assisted right colectomy compared with laparoscopic surgery: A systematic review and meta-analysis. Asian J Surg 2019;42:589-98. [Crossref] [PubMed]
- Duraes LC, Steele SR, Camargo MGM, et al. Conversion to open from laparoscopic colon resection is a marker for worse oncologic outcomes in colon cancer. Am J Surg 2019;217:491-5. [Crossref] [PubMed]
- Trastulli S, Desiderio J, Farinacci F, et al. Robotic right colectomy for cancer with intracorporeal anastomosis: short-term outcomes from a single institution. Int J Colorectal Dis 2013;28:807-14. [Crossref] [PubMed]
- DeSouza A, Domajnko B, Park J, et al. Incisional hernia, midline versus low transverse incision: what is the ideal incision for specimen extraction and hand-assisted laparoscopy? Surg Endosc 2011;25:1031-6. [Crossref] [PubMed]
- Park SY, Choi GS, Park JS, et al. Robotic-assisted transabdominal intersphincteric resection: a technique involving a completely abdominal approach and coloanal anastomosis. Surg Laparosc Endosc Percutan Tech 2013;23:e5-10. [Crossref] [PubMed]
- Ricci C, Casadei R, Alagna V, et al. A critical and comprehensive systematic review and meta-analysis of studies comparing intracorporeal and extracorporeal anastomosis in laparoscopic right hemicolectomy. Langenbecks Arch Surg 2017;402:417-27. [Crossref] [PubMed]
- Benlice C, Stocchi L, Codtedio MM, et al. Impact of the spesific extraction site location on the risk of incisional hernia after laparoscopic colorectal resection. Dis Colon Rectum 2016;59:743-50. [Crossref] [PubMed]
- Brady MT. The advantage of intracorporeal techniques. Ann Laparosc Endosc Surg 2019;4:12. [Crossref]
- Feroci F, Lenzi E, Garzi A, et al. Intracorporeal versus extracorporeal anastomosis after laparoscopic right hemicolectomy for cancer: a systematic review and meta-analysis. Int J Colorectal Dis 2013;28:1177-86. [Crossref] [PubMed]
- Ibáñez N, Abrisqueta J, Luján J, et al. Isoperistaltic versus antiperistaltic ileocolic anastomosis. Does it really matter? Results from a randomised clinical trial (ISOVANTI). Surg Endosc 2019;33:2850-7. [Crossref] [PubMed]
- Formisano G, Misitano P, Giuliani G, et al. Laparoscopic versus robotic right colectomy: technique and outcomes. Updates Surg 2016;68:63-9. [Crossref] [PubMed]
- Deutsch GB, Sathyanarayana SA, Gunabushanam V, et al. Robotic vs. laparoscopic colorectal surgery: an institutional experience. Surg Endosc 2012;26:956-63. [Crossref] [PubMed]
- de'Angelis N, Lizzi V, Azoulay D, et al. Robotic versus laparoscopic right colectomy for colon cancer: analysis of the initial simultaneous learning curve of a surgical fellow. J Laparoendosc Adv Surg Tech A 2016;26:882-92. [Crossref] [PubMed]
- Lujan HJ, Maciel VH, Romero R, et al. Laparoscopic versus robotic right colectomy: A single surgeon's experience. J Robot Surg 2013;7:95-102. [Crossref] [PubMed]
- Isik O, Gorgun E. How Has the Robot Contributed to Colon Cancer Surgery? Clin Colon Rectal Surg 2015;28:220-7. [Crossref] [PubMed]
- Keller DS, Senagore AJ, Lawrence JK, et al. Comparative effectiveness of laparoscopic versus robot-assisted colorectal resection. Surg Endosc 2014;28:212-21. [Crossref] [PubMed]
- Petrucciani N, Sirimarco D, Nigri GR, et al. Robotic right colectomy: a worthwhile procedure? Results of a meta-analysis of trials comparing robotic versus laparoscopic right colectomy. J Minim Access Surg 2015;11:22-8. [Crossref] [PubMed]
- Shin JY. Comparison of short-term surgical outcomes between a robotic colectomy and a laparoscopic colectomy during early experience. J Korean Soc Coloproctol 2012;28:19-26. [Crossref] [PubMed]
- Rawlings AL, Woodland JH, Vegunta RK, et al. Robotic versus laparoscopic colectomy. Surg Endosc 2007;21:1701-8. [Crossref] [PubMed]
- deSouza AL, Prasad LM, Park JJ, et al. Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum 2010;53:1000-6. [Crossref] [PubMed]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis 2009;11:354-64; discussion 364-5. [Crossref] [PubMed]
- Gorgun E, Benlice C. Complete robotic mesocolic excision for right-sided colon cancer - a video vignette. Colorectal Dis 2017;19:949-50. [Crossref] [PubMed]
- Cengiz TB, Aytac E, Gorgun E. Robotic complete mesocolic excision with high vascular tie and intracorporeal side-to-side anastomosis with omental pedicle flap - a video vignette. Colorectal Dis 2018;20:453-4. [Crossref] [PubMed]
- Ozben V, Aytac E, Atasoy D, et al. Totally robotic complete mesocolic excision for right-sided colon cancer. J Robot Surg 2019;13:107-14. [Crossref] [PubMed]
- Spinoglio G, Bianchi PP, Marano A, et al. Robotic Versus Laparoscopic Right Colectomy with Complete Mesocolic Excision for the Treatment of Colon Cancer: Perioperative Outcomes and 5-Year Survival in a Consecutive Series of 202 Patients. Ann Surg Oncol 2018;25:3580-6. [Crossref] [PubMed]
- Ozben V, Baca B, Atasoy D, et al. Robotic complete mesocolic excision for right-sided colon cancer. Surg Endosc 2016;30:4624-5. [Crossref] [PubMed]
- Miller PE, Dao H, Paluvoi N, et al. Comparison of 30-day postoperative outcomes after laparoscopic vs robotic colectomy. J Am Coll Surg 2016;223:369-73. [Crossref] [PubMed]
- Tong DKH, Law WL. Laparoscopic versus open right hemicolectomy for carcinoma of the colon. JSLS 2007;11:76-80. [PubMed]
- Parisi A, Scrucca L, Desiderio J, et al. Robotic right hemicolectomy: Analysis of 108 consecutive procedures and multidimensional assessment of the learning curve. Surg Oncol 2017;26:28-36. [Crossref] [PubMed]
Cite this article as: Tatar C, Cengiz TB, Gorgun E. What does robotic right colectomy add to its laparoscopic counterpart? Ann Laparosc Endosc Surg 2020;5:2.