
High-resolution manometry findings with hiatus hernia
Introduction
High-resolution manometry (HRM) is usually recommended as a part of the evaluation of gastroesophageal reflux disease (GERD) when a procedural intervention is under consideration, be that a fundoplication, Linx implant, or transoral incisionless fundoplication. Among the objectives of that evaluation is an assessment of the integrity of the esophagogastric junction (EGJ) as an antireflux barrier including the characterization of an axial hiatal hernia. However, there are numerous potential pitfalls in that assessment and consensus is lacking on how to interpret the complex intraluminal pressure signature of both the normal and anatomically disrupted EGJ. Among the technical difficulties encountered are the effects of respiratory variation, the effect of movement of the pressure sensor relative to the sphincter with breathing and swallowing, the recording fidelity of the sensor, distinguishing an intrinsic contraction from extrinsic compression, the effects of pulsations from the heart and aorta, and the extreme radial asymmetry and temporal variability of the EGJ itself. It is from this vantage point that the HRM findings of both the normal EGJ and type I (sliding) hiatus hernia must be explored.
Normal EGJ pressure morphology: teasing apart the 3D-HRM signal
High among the difficulties encountered in interrogating the EGJ pressure profile are limitations of the pressure sensors themselves. Historically, relatively few sensors were employed, and one needed to repeatedly reposition the manometric catheter or actually pull it across the EGJ to obtain an axial pressure profile. However, with the advent of HRM and the interpolation paradigms that transform HRM into esophageal pressure topography plots, such things are of historical interest only. There is no going back to those archaic techniques. None the less, understanding the HRM pressure signature of the EGJ in a pressure topography format can be quite challenging as it bears no resemblance to recordings obtained with line tracings from 3–8 widely spaced pressure sensors. Adding to the difficulty is that with the current HRM catheter designs each of the closely spaced sensors is circumferentially sensitive such that if it is contacted on one side it can yield a pressure signal that is indistinguishable from the circumferential squeeze of a sphincter. This has profound implications with respect to the EGJ because that pressure signal is comprised of both an extrinsic crural diaphragm (CD) and intrinsic lower esophageal sphincter (LES) component, each of which is subject to independent physiological control mechanisms and pathophysiology. An experimental approach to unraveling this complexity was to develop a 3D HRM device that preserved rather than averaged the circumferentially unequal pressures (1,2). Figure 1 is an illustration of that device comprised of a 3D segment intended to interrogate the EGJ as well as ‘conventional’ HRM sensors both proximal and distal to it to record esophageal and intragastric pressure respectively. All told, the 3D-HRM device monitors 128 independent pressure signals in real time, 96 of which are focused on the EGJ. Although this device is not commercially available, recordings obtained from it are very informative with respect to understanding the appearance of recordings obtained with standard HRM devices.
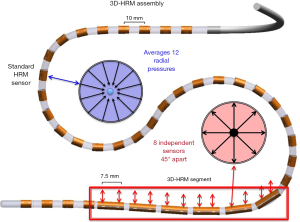
Figure 2 is a sagittal view of an MRI scan in a normal individual selected for illustration because the CD and esophagus outlined in orange and green respectively were both readily identifiable. Note that the axis of the CD is not perpendicular to the axis of the esophagus; rather, it is at about 58° in this example. Consequently, its pressure signature will be oblique with respect to the axis of the esophagus. Inserted into the esophagus is a 3D HRM catheter with the 3D segment depicted in yellow and the remainder in red. Now consider the cylindrical representation of the 3D segment recording at inspiration inserted onto the MRI. Brighter colors on the cylinder as well as outward deflection depict greater pressure. Note that the CD signal (blue arrow) is the most intense pressure recorded but is largely restricted to the anterolateral wall of the esophagus. Its counterpart, the green arrow is temporally synchronized with it on the opposite wall and likely reflects contact of the HRM device with the opposing wall of the esophagus.

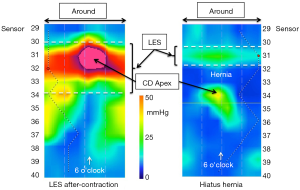
Although the cylindrical representation of the 3D-HRM signal is illustrative, it is difficult to interrogate quantitatively because half or the signals are hidden and the whole recording cannot be viewed without rotating the cylinder. Hence, Figure 3 shows the technique of unfolding the cylinder and depicting the 3D signal as a rectangle centered on the CD apex as defining the 6 o’clock orientation. When imaged in real time, the CD apex intensifies and then de-intensifies with inspiration and expiration respectively. In fact, the CD signal is so dominant that one wonders where the LES is in Figure 3. A key attribute of the LES is that, being a product of circular (or slightly spiral) muscle contraction, it is a circumferential pressure perpendicular to the axis of the esophagus and anatomically localized within the hiatus. However, it is impossible to localize the LES in areas where the pressure topography is obscured by the superimposed CD, so its margins can only be identified in the radial sectors not affected by the CD pressure recording. The horizontal white dashed lines approximate the margins of the LES in Figure 3. Note that with the sensors spaced 7.5 mm apart this implies that the intrinsic LES is about 2 cm long, which is consistent with physiological data. Support for this interpretation of the Figure 3 pressure topography is in Figure 4. The left panel of Figure 4 shows the pressure topography during the LES after-contraction following a swallow; this intensity gradually dissipates over the ensuing 5–10 seconds. The panel on the right in Figure 4 is a recording from a subject with a hiatal hernia wherein the CD is not at all superimposed on the LES and the individual pressure signatures of the LES and CD can be seen. Both are weak, consistent with this individual having an incompetent EGJ, but the pressure signatures of the LES and CD are nonetheless preserved.

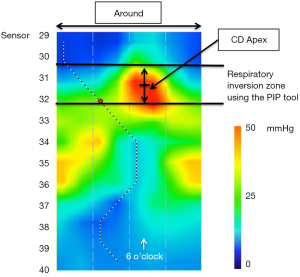
Another manometric landmark pertinent to the EGJ is the respiratory inversion point (RIP), also referred to as the pressure inversion point (PIP). The RIP is the location along the axis of the esophagus at which the inspiratory signal transitions from being a pressure increase, characteristic of the abdomen, to a pressure decrease, characteristic of the chest. HRM analysis software (Manoview, Medtronic Inc, Minneapolis, MN) localizes the RIP using the ‘PIP tool’ which allows the user to scroll up and down over the EGJ pressure complex and find the location at which the pressure signals 1 cm above and 1 cm below a given location most nearly cancel each other out. In essence, the inspiratory increase one cm below that point is negated by the inspiratory decrease one cm above it. The RIP is really more of a zone than an exact point and as illustrated in the 3D-HRM plot shown in Figure 5, the RIP zone normally localizes with the CD-apex signal. This is important as we transition to discussing conventional HRM because it provides a mechanism for localizing the CD-apex even when its radially asymmetric signature is obscured by circumferential pressure averaging.
Normal EGJ pressure morphology in HRM
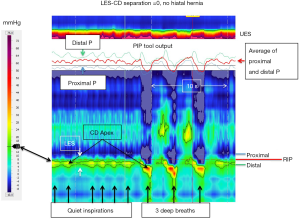
While a strength of 3D-HRM lies in its ability to preserve circumferential pressure asymmetry, a strength of conventional HRM lies in its ability to depict a seamless longitudinal profile of intraesophageally pressure change over time in a single image; this requires a movie with the 3D format. With conventional HRM, the axis used in 3D to depict the radial origin of a pressure is used to show temporal change of a single pressure sensor over time making respiratory changes and vascular pulsations evident by their synchrony with breathing and pulse rate respectively. Figure 6 illustrates a normal EGJ during quiet respiration including a segment depicting 3 deep inspirations and an illustration of the PIP tool isolating the RIP, indicative of the location of the CD-apex. The black line indicating the 15 mmHg isobaric contour (referenced to atmospheric pressure) delineates the greatest pressure regions of the LES and CD, which completely overlap in this example with no hiatal hernia. The PIP tool is used to identify the RIP which localizes toward the upper margin of the CD-apex signal (see figure legend for detailed explanation). Refer back to Figure 3 to conceptualize how the LES and CD contributions to the dynamic EGJ pressure profile in Figure 6 recognizing that any area of pressure increase in Figure 3 becomes a band of pressure, fluctuating with respiration, in Figure 6.
Hiatus hernia in HRM, i.e., LES-CD separation >1 cm
With a type I (sliding) hiatus hernia there is progressive anatomical disassociation between the CD and the LES attributable to laxity of the phrenoesophageal ligament and intermittent or permanent displacement of the LES into the mediastinum. Figure 7 illustrates a recording obtained from an individual with a small hiatal hernia, evident by an LES-CD separation of 2 cm. In HRM, this becomes evident with axial separation between the CD and LES signals on the pressure topography plot. EGJ morphology is characterized as type I, II, or III in the Chicago Classification v3.0 (3,4); type 1 with superimposed LES and CD, type 2 with axially separated LES and CD pressure signals separated by less than 2 cm, and type 3 with a ≥2 cm separation between the LES and CD pressure signatures. That classification will likely not survive future iterations of the Chicago Classification with the focus instead shifting to whether or not the LES-CD separation exceeds 1 cm (indicative of hiatus hernia) and whether or not the CD-apex pressure signal continues to define the locus of the RIP (indicative of the competence of the CD as a extrinsic sphincter).
In its early stages, a type I hernia is difficult to distinguish from normal, appearing only as an exaggeration of the normal phrenic ampulla on fluoroscopy and a partially overlapping LES and CD on HRM. Furthermore, EGJ pressure morphology can vary over time, even within a single patient study, transitioning between superimposed and separated CD and LES components (5) suggesting that with laxity of the phrenoesophageal ligament the degree of axial separation between the LES and CD can vary in the course of a day based on factors such as patient position or breathing. When such variation is seen, it makes sense to score it as the maximal value (or to report the range), the critical question being whether or not an anatomical hiatal hernia exists. HRM-defined LES-CD separation has been shown to correlate closely with the presence or absence and size of hiatus hernia as determined by endoscopy or barium x-ray with sensitivity and specificity of 88% and 95% respectively (6). LES-CD separation also correlates with reflux severity as determined by pH-metry (3,7,8). True, the Weijenborg et al.’s 2015 analysis (6) begs the question of which test is the most appropriate reference standard for detecting hiatal hernia because the unequivocal diagnosis of hiatus hernia is ultimately made intraoperatively wherein the spatial relationship between the EGJ and CD and presence or absence of a hernia sac are visually evident. Along that line, a recent analysis compared the accuracy of HRM, endoscopy, and barium radiography to surgery in detecting and sizing hiatus hernia (9). That analysis concluded that HRM, using the LES-CD metric, outperformed the other modalities with a sensitivity of 94%, specificity of 92% and kappa value of 0.85. In that analysis, HRM reached both optimal sensitivity and specificity for detecting hiatus hernia with a threshold LES-CD separation of 1.2 cm. For simplicity, make this 1 cm because, after all, the spatial resolution of a device that measures pressures at 1 cm intervals is 1 cm regardless of how many decimal places the computer spits out.
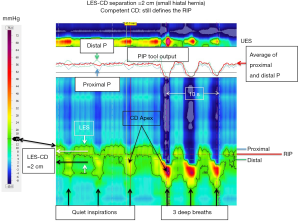
The RIP
As the LES and CD become spatially separate there is the added issue of whether the RIP remains associated with the CD signal in the EGJ pressure complex or not. Although the precise physiological meaning of the RIP in this context is uncertain, there can be general agreement regarding the observations that: (I) the RIP can never be below the diaphragm; (II) when the CD is superimposed on the LES (i.e., LES-CD separation <1 cm), the RIP localizes above the EGJ pressure complex placing the LES physiologically within and beneath the diaphragmatic hiatus; and (III) with spatial separation of the CD and LES (i.e., LES-CD separation >1 cm), the RIP can localize either at the CD (as in Figure 7) or above the CD component placing it either within the hernia or at the LES as in Figure 8. Supporting the relevance of this distinction, a recent analysis exploring the relevance of hiatal hernia pressure topography subtyped individuals with LES-CD separation as ‘B’ or ‘C’ depending on whether the RIP localized above or below the LES respectively (10). In that analysis, subtype B was less likely to exhibit pathological reflux on pH-metry than subtype C. The authors interpreted this to support the contention that subtype B was indicative of the LES remaining within the abdominal compartment and being advantageous. Of note, the patients associated with Figures 7,8 both had clear-cut reflux disease. In the case of Figure 7 they had Barrett’s esophagus with high-grade dysplasia and in the case of Figure 8 a Bravo pH-metry study found pathological reflux on four out of four days with an average esophageal acid exposure time of 10%.
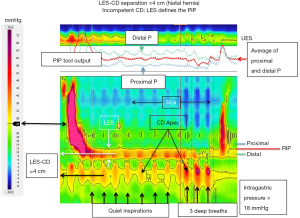
Interpreting the distinction between a competent and an incompetent CD somewhat differently, with greater degrees of LES-CD separation (i.e., >2 cm), there is the additional factor of whether or not the CD effectively compartmentalizes the stomach from the herniated stomach during inspiration. When it does, it is exhibiting greater sphincteric function than when it doesn’t, presumably because the hiatal aperture is less dilated (11). Although not precisely addressing this distinction, evidence supporting the relevance of CD competence comes from a logistic regression model of barrier function that simultaneously examined expiratory LES pressure, LES-CD separation, and inspiratory EGJ augmentation while controlling for age and BMI. In that analysis, only inspiratory augmentation had a significant independent association with GERD as defined by pH-metry (3).
Conclusions
Accepting that the presence and size of hiatus hernia is a clinically relevant measurement, it can be concluded that there is strong evidence supporting that LES-CD separation >1 cm evident during quiet respiration during an HRM is indicative of hiatus hernia. With respect to the localization of the RIP, there is less agreement on its clinical significance, but in instances of the LES-CD separation exceeds 1 cm the RIP can localized at or above the CD component. Hence the three possible EGJ morphologies are: (I) no hiatus hernia (LES-CD separation <1 cm); (II) hiatus hernia with a competent crural diaphragm (LES-CD separation >1 cm with the RIP at the CD level); and (III) hiatus hernia with an incompetent CD (LES-CD separation >1 cm with the RIP above the CD component placing it either within the hernia or at the LES). The clinical significance of a competent vs. incompetent CD is a matter of continued debate.
Acknowledgments
Funding: The study was supported by R01 DK092217 (John E. Pandolfino) from the US Public Health Service.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.03.08). PJK consulting for Ironwood, Bayer. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kwiatek MA, Pandolfino JE, Kahrilas PJ. 3D-high resolution manometry of the esophagogastric junction. Neurogastroenterol Motil 2011;23:e461-9. [Crossref] [PubMed]
- Nicodème F, Lin Z, Pandolfino JE, Kahrilas PJ. Esophagogastric junction pressure morphology: comparison between a station pull-through and real-time 3D-HRM representation. Neurogastroenterol Motil 2013;25:e591-8. [Crossref] [PubMed]
- Pandolfino JE, Kim H, Ghosh SK, et al. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol 2007;102:1056-63. [Crossref] [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Bredenoord AJ, Weusten BL, Timmer R, et al. Intermittent spatial separation of diaphragm and lower esophageal sphincter favors acidic and weakly acidic reflux. Gastroenterology 2006;130:334-40. [Crossref] [PubMed]
- Weijenborg PW, van Hoeij FB, Smout AJ, et al. Accuracy of hiatal hernia detection with esophageal high-resolution manometry. Neurogastroenterol Motil 2015;27:293-9. [Crossref] [PubMed]
- Ham H, Cho YK, Lee HH, et al. Esophagogastric junction contractile integral and morphology: Two high-resolution manometry metrics of the anti-reflux barrier. J Gastroenterol Hepatol 2017;32:1443-9. [Crossref] [PubMed]
- Tolone S, de Cassan C, de Bortoli N, et al. Esophagogastric junction morphology is associated with a positive impedance-pH monitoring in patients with GERD. Neurogastroenterol Motil 2015;27:1175-82. [Crossref] [PubMed]
- Tolone S, Savarino E, Zaninotto G, et al. High-resolution manometry is superior to endoscopy and radiology in assessing and grading sliding hiatal hernia: a comparison with surgical in vivo evaluation. United European Gastroenterol J 2018;6:981-9. [Crossref] [PubMed]
- Akimoto S, Singhal S, Masuda T, et al. Classification for esophagogastric junction (EGJ) complex based on physiology. Dis Esophagus 2017;30:1-6. [Crossref] [PubMed]
- Kumar D, Zifan A, Ghahremani G, et al. Morphology of the esophageal hiatus: is it different in 3 types of hiatus hernias. J Neurogastroenterol Motil 2020;26:51-60. [Crossref] [PubMed]
Cite this article as: Kahrilas PJ. High-resolution manometry findings with hiatus hernia. Ann Laparosc Endosc Surg 2021;6:5.


