Single anastomosis duodenal switch: a novel procedure for obesity and metabolic surgery
Introduction
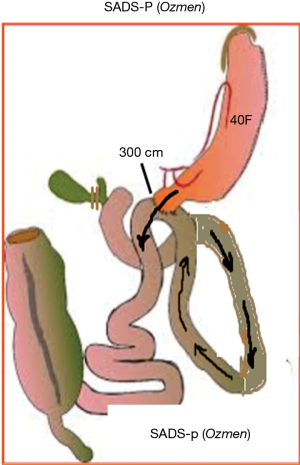
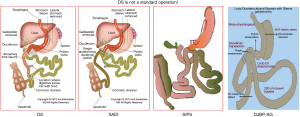
Biliopancreatic diversion (BPD) was introduced by Scapinaro et al. and consisted of a distal gastrectomy with a long roux-n-Y reconstruction. In this operation entero-enterostomy is placed at 50 cm from the iliocecal valve (1). Scapinaro’s procedure was modified by Hess into the BPD-duodenal switch (DS) (2). Hess proposed a vertical sleeve gastrectomy (SG) and also modified the length of the common channel to 100 cm (2). This procedure considered as one of the best and effective procedure for the treatment of obesity and metabolic disorders (3). As the procedure itself is very complex and difficult, for worldwide use with minimally invasive techniques, it was simplified. Sánchez-Pernaute et al. described single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) in 2007 (4). In their technique, preservation of pylorus eliminates the bile reflux therefore bile diversion is unnecessary. Since then they modified the technique and many other modifications have been developed including Cottam, Huang, Lee and Ozmen (5-8) (Figures 1,2).
I introduced the technique of single anastomosis duodenal switch (SADS) and performed over hundred procedures with success on weight-loss and metabolic control (8,9). The present paper explains the steps of the procedure for both laparoscopic and robotic approaches.
Surgical technique
I will explain the technique for both laparoscopic and robotic approach in ten steps.
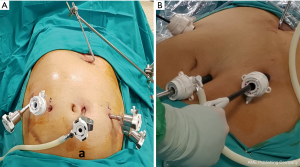
- Procedure will start with the positioning of the patient for which we prefer French position (supine position with the legs spread apart and surgeon stands between legs). The operating table could be tilted in 30° reverse Trendelenburg position as the surgeon needs. Insufflation is usually performed from the first trocar insertion site. It can be over or at the umbilical region of the patient. However, there are two pitfalls during the establishment of the pneumoperitoneum. The first important point is that obese individuals have a wider upper abdomen, and if the initial trocar is placed low near the umbilicus, the dissection of the upper abdomen can be more difficult. The second pitfall is bypassing the falciform ligament. In obese individuals, the falciform ligament can be hypertrophied due to adipose tissue hyperplasia. If insufflation is to be carried out near the midline, the falciform ligament can make insufflation and initial trocar insertion technically challenging. If the procedure is being done laparoscopically four trocars including the liver retractor will usually be enough. However, if any difficulty in dissection is encountered, then, right and left lateral hypochondriac region can be used to insert 5 mm port for traction and exposure purposes. If the procedure is being done robotically then 5 to 6 trocars will be used including the liver retractor. Trocar placements for both robotic (using daVinci Xi) and laparoscopic approaches are shown in the Figure 3.
- After abdominal exposure, If possible, cholecystectomy should be the first. Following cholecystectomy, before mobilization of the greater curve oesophagogastric junction (OGJ) should always be checked to make sure that there is no hiatal hernia and then the fundus is released.
- Mobilization of greater curve might start from the mid-corpus and first go upwards to OGJ. During this stage attention should be paid to the short-gastric vessels especially superior gastrosplenic vein. Surgeon should not hesitate to clip if it is visible! The mobilization continues further down to the gastroduodenal artery or 3 cm from the pylorus (whichever comes first). In order to avoid any bleeding or tears meticulous and careful dissection is necessary around duodenum.
- After mobilization, duodenum is transected using stapling device with 45 mm blue cartridge. After transection procedure continues with gastric resection to create a loose sleeve with the bougie size 42 and over. If you are using 36 F bougie for standard sleeve then staple line should stay 1 cm away from the 36 F bougie. Till this step, procedure is similar to SADI-S.
- We count the small bowel starting from the Treitz ligament and it should always be kept in mind that it is very important to count whole length of the bowel. Three hundred cm in length of jejunum should be measured and fixed to the duodenum using continue vicryl suture which might be used as the posterior layer of the anastomosis.
- Then, 1 cm holes are created on both sides (duodenotomy and jejunotomy) for the anastomosis and nasogastric tube is passed to efferent loop in this stage.
- Single layer anastomosis is done using pre-tied 3/0 V-lock suture. Extra sutures might be inserted on the anterior wall especially at the corners. Leak test using methylene blue is always done to control the anastomosis and the bowel loops.
- SG material is removed through the 12 mm trocar on the left side (during laparoscopic) or from the assistant port (during robotic).
- We always insert a drain to the subhepatic area.
- After completing all these steps, trocars are removed and trocar sites are closed following desufflation.
Discussion
SADS is the modification of standard BPD/DS and there are many modifications of SADS also (10). Metabolic and weight-loss outcomes of SADI/SIPS techniques are known (4,5,10,11). However, the certain amount of the patients suffer from malnutrition and GI symptoms such as diarrhea after SADI or SIPS (10,11). There is a clear need of alternative procedures with fewer side-effects.
We first introduced our approach as an alternative to SADI/SIPS in 2014 and presented first 13 laparoscopic cases during ELSA congress in 2015 and then again in ELSA congress in 2017, I gave a talk on robotic SADS: proximal approach (8). Since we performed the first case, we have used this technique either laparoscopic or robotic in more than hundred cases with morbid obesity and diabetes and results were partly presented elsewhere (9). Results shown in Table 1 includes total of 1,349 patients with 2 years follow-up. Patients with the median [range] age of 37 [22–69] years with BMI of 49 [41–73] kg/m2 underwent DS. Eighty-two patients had type 2 diabetes with a median [range] of 45 [3–100] months with 8.6% (4.9–9.9%) of HbA1C levels. After surgery HbA1C levels decreased to 5.1% (4.8–5.6%) in 12 months (remission rate of 100%). The procedure seems to be effective for both weight-loss and metabolic control for type 2 diabetes especially in patients with high BMI. In our practice we found that robotic approach is better in those patients as it creates better view, better maneuver ability and give chance to surgeon in order to do the difficult jobs in a narrow space.
Table 1
| Procedure | No | Age (years) | BMI (kg/m2) | T2DM (no) | DM-duration (months) | Remission (%) |
|---|---|---|---|---|---|---|
| SG | 682 | 40 [18–66] | 42 [35–57] | 158 | 21 [3–60] | 75 |
| MGB/OAGB | 567 | 50 [26–62] | 54 [42–69] | 315 | 68 [3–240] | 93 |
| SADS | 100 | 37 [22–69] | 49 [41–73] | 82 | 45 [3–100] | 100 |
SG, sleeve gastrectomy; MGB/OAGB, mini-gastric bypass/one anastomosis gastric bypass; SADS, single anastomosis duodenal switch; T2DM, type 2 diabetes mellitus.
We also studied the fecal elastase levels in order to check exocrine pancreatic function after surgery (12). We compared levels after SG, mini-gastric bypass/one anastomosis gastric bypass (MGB/OAGB) and SADS. We concluded that almost every patients after gastric bypass or DS are suffering from pancreatic exocrine insufficiency, whereas only 7–14% are having symptoms of malnutrition (4,10). Although there are many modifications, except for one study with 5 years follow-up, follow up data remains limited to 1 year which means more patients might suffer from malnutrition, weight gain and metabolic failure.
Therefore in patients with the symptoms, pancreatic enzyme replacement therapy might be the solution (12).
Conclusions
SADS is technically easier and has fever complications, shorter hospital stay and similar metabolic effects with standart DS. Our technique (SADS-Ozmen modification) with 2 years follow up seems better than other modifications in terms of complications and metabolic effects.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mehmet Mahir Ozmen) for the series “Bariatric and Metabolic Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-2019-bms-07). The series “Bariatric and Metabolic Surgery” was commissioned by the editorial office without any funding or sponsorship. MMO served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Mar 2019 to Feb 2021.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Scopinaro N, Gianetta E, Civalleri D, et al. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg 1979;66:618-20. [Crossref] [PubMed]
- Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg 1998;8:267-82. [Crossref] [PubMed]
- Hess DS, Hess DW, Oakley RS. The biliopancreatic diversion with the duodenal switch: results beyond 10 years. Obes Surg 2005;15:408-16. [Crossref] [PubMed]
- Sánchez-Pernaute A, Rubio Herrera MA, Pérez-Aguirre E, et al. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg 2007;17:1614-8. [Crossref] [PubMed]
- Cottam A, Cottam D, Roslin M, et al. A Matched Cohort Analysis of Sleeve Gastrectomy With and Without 300 cm Loop Duodenal Switch With 18-Month Follow-Up. Obes Surg 2016;26:2363-9. [Crossref] [PubMed]
- Huang CK, Goel R, Tai CM, et al. Novel metabolic surgery for type II diabetes mellitus: loop duodenojejunal bypass with sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech 2013;23:481-5. [Crossref] [PubMed]
- Lee WJ, Lee KT, Kasama K, et al. Laparoscopic single-anastomosis duodenal-jejunal bypass with sleeve gastrectomy (SADJB-SG): short-term result and comparison with gastric bypass. Obes Surg 2014;24:109-13. [Crossref] [PubMed]
- Ozmen MM, Sahin TT, Ozen S, et al. Laparoscopic Proximal Duodeno-Jejunal Bypass With Sleeve Gastrectomy (Pdbsg). Daegu: ELSA Congress, Oral Presentation/FP26-2, 2015.
- Ozmen MM, Guldogan EC, Gundogdu E. Changes in HOMA-IR Index After Bariatric Surgery: Comparison of SADS-p and MGB-OAGB. Surg Obes Relat Dis 2018;14:S109. [Crossref]
- Topart P, Becouarn G. The single anastomosis duodenal switch modifications: a review of the current literature on outcomes. Surg Obes Relat Dis 2017;13:1306-12. [Crossref] [PubMed]
- Sánchez-Pernaute A, Ángel Rubio Herrera M, Antona EM, et al. Single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S). Absolute results at 5 years. Surg Obes Relat Dis 2016;12:S70-1. [Crossref]
- Ozmen MM, Gundogdu E, Guldogan EC, et al. Effect of Bariatric Surgery on Exocrine Pancreatic Function. Surg Obes Relat Dis 2018;14:S103. [Crossref]
Cite this article as: Ozmen MM. Single anastomosis duodenal switch: a novel procedure for obesity and metabolic surgery. Ann Laparosc Endosc Surg 2020;5:25.