
Transanal Surgery for benign tumor or early rectal cancer: state of the art and future prospects of the surgeon
Introduction
Transanal surgery for rectal polyps and villous adenomas is a widely used surgical technique. Conventional transanal excision (TAE) is the most common technique, with excellent results in terms of morbidity but also recurrence rate, knows as Parks’ procedure (1). Technical advances have made it possible to develop new devices that facilitate surgery and that allow local excision for larger tumors and more difficult to access. For example, transanal endoscopic operation (TEO) described by Buess et al. at the end of the eighties (2) offers the advantage of better visibility and better exposure.
The most used endoscopic technique, the endoscopic mucosal resection (EMR) raises the question of a piecemeal resection without a good staging. Endoscopic submucosal dissection (ESD) is an emerging endoscopic technique that could have comparable results to transanal surgery. The lack of comparative studies leaves the debate open.
Classically, Transanal surgery was done for early rectal tumors, the important morbi-mortality rate of radical rectal surgery and impairment of quality of life led the surgeon to reconsider management and to adapt neoadjuvant strategy in order to transform major surgery in tumorectomy. Transanal endoscopic surgery could be a preferred option in selected cases.
This review describes the different procedures to perform a transanal surgery, in comparison with endoscopic techniques, and discuss indications: benign tumor including large villous polyps, early rectal cancer. We also present the concept of organ preservation in rectal cancers after neoadjuvant chemoradiotherapy and its surgical and oncologic results.
Transanal surgery: which operative technique?
Technical evolution and description of the procedures
Different technique have been described such as the Kraske (1885) transsacral approach (3,4) or the York-Mason (1977) transsphincteric technique (5) but conventional TAE of a rectal polyp or early rectal cancer is most often carried out according to the Parks’ technique describing since 1973 a local excision through the anal canal (1). This technique is now well codified (6) and has long been the most used. TAE procedure consists in a full-thickness excision after locating the tumor using for example a Parks’ retractor for exposition. A line is drawn all around the lesion using electrocautery, before starting the resection, to ensure a free margin of 1 cm. En-bloc full-thickness excision, exposing the fat, is a key point to ensure resection of the entire rectal wall for both staging and curative treatment. The main limit of the conventional TAE technique concerns highest tumors, inaccessible, generally located more than 7-8 cm from the anal verge.
Buess et al. published in 1988 an innovative transanal endoscopic microsurgery (TEM) technique to break free from the limits of the TAE and to avoid the invasive York-Mason approach (2). To perform TEM, a rectoscope is introduced for gas insufflation in the rectal cavity. TEM offers many advantages, with a magnified view to perform an en-bloc full-thickness excision. Specimen is then stretched and pinned on a cork plate for pathologic exam. The surgical outcomes of the first 140 patients were very encouraging with a low complication rate of 5% with only 1 (0.7%) postoperative bleeding. Concerning oncologic outcomes, 12 patients had local resection only for a pT1 carcinoma and there was no local recurrence or tumour spread (7). Buess et al. was already describing the need for a learning curve for this difficult technique, with the implementation of a training program (8). In the original technique, Buess et al. suggested to close the defect with a continuous resorbable suture finished using a silver clip on the thread (2). But this point remains controversial as the defect is below the peritoneal reflexion. To date, only 2 randomized control trial (RCT) (9,10) and 5 retrospective comparative studies (11-15) compared outcomes according to the closure of the rectal wall. Table 1 summarizes these results. There appears to be a trend towards closing the defect in terms of postoperative complications but the result was not confirmed in the RCTs. A recent meta-analysis found no significant difference about morbidity, postoperative infection, postoperative bleeding rate and reintervention rate (16). So the discussion about closing the rectal wall after TEM is still open and no recommendation could be made. A technical issue that could arise with TME and didn’t exist with the TAE is the occurrence and management of peritoneal perforation, in 1.7% to 15% of cases (17-21), particularly for anterior and upper rectal tumors. However, this complication should not cause the technique to be discussed because its laparoscopic suture is feasible as showed by Mege et al., without an increase in postoperative morbidity rate if diagnosis was made during the procedure (18).
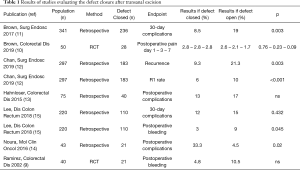
Full table
Comparison of operative techniques
Compared to TAE, TEM has proven its superiority (Table 2). Comparatives studies found advantages for TEM in terms of negative resection margin, fragmentation of the specimen (no en-bloc resection), postoperative morbidity and local recurrence rate (22-25). Clancy et al. published a meta-analysis with these data (26). They also demonstrated the superiority of TEM for negative microscopic margin rate (P<0.001), specimen fragmentation rate (P<0.001) and recurrence (P<0.001).
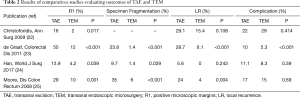
Full table
Different devices are used to perform TEM, with a better visualization and precision than TAE. Buess et al. initially described a reusable rectoscope still in use today: TEO® platform (Karl Storz, Tuttlingen, Germany) (2). Several disposable materials also allow TEM through a single-incision laparoscopic surgery port, called Transanal mini-invasive surgery (TAMIS) (27). The two most commonly used are the single-incision assisted laparoscopic surgery (SILS) Port (Covidien, United States) and the GelPOINT Path Transanal Access Platform (Applied Medical, United States). There is no recommendation for preferential use of any of the devices. None of the techniques have proven to be superior for the quality of the surgical resection (21,28). TEO and TAMIS seem feasible depending on the surgeon's habits. Indeed, these surgical techniques require rigorous learning curve to achieve quality oncologic results, in particular the rate of positive margin (R1). Lee et al. published an observational cohort studies showing that TAMIS requires a minimum of 14–24 cases to reach an acceptable R1 resection rate (29).
Technical and material advances have made it possible to increase the feasibility of transanal surgery.
Transanal surgery for benign tumor or early rectal cancer
Rectal polyps and villous tumors
Some indications of transanal surgery for rectal tumors are now well established. Concerning benign tumors, TEM has the advantage of a full-thickness resection compared with EMR, which generally performs a piecemeal resection, especially for large villous tumors that have an important risk of malignant transformation (30), up to 33% of unsuspected cancer for giant villous adenomas of the rectum (31). The difficulty in these cases of large villous tumors remains the preoperative evaluation even with careful clinical examination and MRI. For benign adenomas and villous tumors, TEM achieve a complete staging contrary to the EMR, with good surgical and oncologic outcomes. A large Italian multicentric cohort of 588 patients with benign tumor reported a global morbidity rate of 11.4%, no postoperative mortality, a percentage of local recurrence of 4.3% with a median operative time of 105 min (32). Concerning villous tumors, Pigot et al., in a French series of 207 consecutive patients with large rectal villous adenomas (mean size of resected tumor: 5.4 cm), showed excellent results with a recurrent rate of 3.6% with a mean follow-up of 74 months. They noticed that specific recurrence-free probability was 99.5 percent at one year, 96 percent at five years, and 95 percent at ten years. (30).
Primary surgery for early rectal cancer
Management of early rectal cancer that can be discovered on the pathologic report or diagnosed at the beginning of treatment must take into account many parameters in particular the risk of lymph node involvement. Depth invasion of the rectal wall need to be described. The rate of lymph node involvement varies from 0 to 15% for T1 tumors and from 16 to 28% for T2 (33). Kikuchi classification for pT1 rectal adenocarcinoma consists in the division of the submucosal layer in three parts: sm1, sm2 and sm3 (34) with a lymph node involvement risk of 0–3% for sm1, 8–10% for sm2, and 23–25% for sm3 (35). The other additional risk factors increasing the rate of lymph node involvement that are details in the recent French Guidelines for the managements of rectal cancer are poor-differentiated tumors, vascular or lymphatic emboli and tumor budding (36-38). Bach et al. described a multivariable analysis to determine predictive factors of local recurrence: depth of invasion, diameter of the tumor and intramural lymphovascular invasion (39). Tumor size and circumference are still debating (33) but are considered to be factors associated with technical difficulty as well as height (upper rectum) (19,40). Positive resection margin is usually considered as a pejorative factor but no study demonstrated the need to achieve a complete lymphadectomy instead of a new local excision (33,36).
A completion proctectomy with total mesorectal excision (cTME) should be discussed for patients with poor prognosis criteria due to lymph node involvement risk and local recurrence rate. In practice, local excision for T1sm1 without further risk factors is acceptable in term of nodal disease and local recurrence but a cTME should be discussed since there are associated risk factors or for T1sm2 or T1sm3 tumors.
Surgical outcomes after TEM for benign tumors or early rectal cancer (with no other treatment, excluding all neoadjuvant therapy) in large series are presented in Table 3 (20,32,39-44). Perioperative mortality is closed to 0 and major complication rate, which correspond to stages III-IV-V of the Dindo-Clavien classification (45), varies to a maximum of 3.8%.
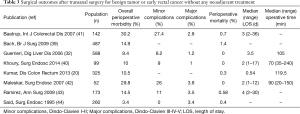
Full table
With such good morbi-mortality results, it is difficult to envisage alternative management for these tumors. The TREND study was a retrospective analysis collecting data from patients treated with TEM or EMR for a large rectal adenoma (>2 cm). Early recurrence rate was 10.2% in the TEM group and 31.0% in the EMR group (P<0.001) (46).
Management of small rectal cancer: surgical resection or ESD?
ESD is an emerging endoscopic treatment, which competes with surgical techniques due to its proven superiority over the EMR in terms of en-bloc and curative resections and local recurrence rate, especially for tumors larger than 2 cm (47). Indeed, this technique allows mucosal and also sub-mucosal resection and avoids piecemeal resection contrary to EMR. But ESD is a complex procedure, responsible for a longer operative time (47). A debate has been developed between surgeons and endoscopists particularly with regard to the operative time, but also to the cost. The first retrospective study published by Park et al. in 2012 comparing ESD and TEM included adenomas with high grade dysplasia or early rectal cancer (T1) (48). En-bloc resection (96.7% vs. 100%, P=0.476) and R0 resection (96.7 vs. 97, P=1) rates did not differ between ESD group and TEM group respectively but procedure time for the ESD group was shorter than that for the TEM group (mean: 84.0 min vs. 116.4 min, P=0.023). There were few patients in the study (only 30 ESD and 33 TEM). Kawaguti et al. published the second retrospective comparative study including 11 ESD and 13 TEM (49). There was no difference in the en-bloc resection rates with free margins: 81.8% vs. 84.6% (P=0.40) in ESD group and TEM group respectively. The operative time was also comparable (P=0.69). Finally a systematic review published by Arezzo et al. in 2014 with 21 series (but no comparative studies) for a total of 2,077 patients showed a higher en-bloc resection (P<0.001) and RO (P<0.001) rates in the benefit of TEM with no difference in the postoperative complications rate (50). So there is a clear lack of quality comparative studies on the subject and there is no consensus for the management of the large benign rectal adenomas or early rectal cancer. In this case, the European Association for Endoscopic Surgery (EAES) recommends that ESD and TEM are the two established techniques to perform local excision (51). A French prospective non-randomized study is in progress to compare ESD with TEM for early rectal cancer and rectal adenomas for R0 resection rate and the cost-effectiveness ratio: MUCEM-GRECCAR 13 (ClinicalTrials.gov Identifier: NCT02885142).
Transanal surgery for rectal cancer following neoadjuvant treatment
Rational
Radical surgery for rectal cancer is based on total mesorectal excision (TME) (52). This management is well codified by oncologic quality criteria (36) but presents some challenges for the surgeon and the patient. First, sphincter preservation is a major issue that could be difficult for ultra-low rectal tumors. Even when sphincter preservation is possible, functional outcomes could be altered, in particular anal continence, by factors such as the type of anastomosis or the height of the tumor and so the height of the anastomosis (53). Sexual and urinary dysfunctions are also major complications for the patient, often underestimated, becoming more and more of a quality of life concern with an increasing number of studies evaluating prevalence, risk factors and management (54). The third key point for the discussion concerns anastomotic complications after TME. Pelvic abscess and anastomotic leakage rates remain high, up to 19% in large series (55-57). Locally advanced rectal cancer are treated first with neoadjuvant treatment (NAT): the reference is a chemoradiotherapy decreasing local recurrence rate (58,59). Tumor response after NAT need to be evaluated and it is a key point for surgical management. Pathologic complete response (pCR) rate after NAT and resection, which corresponds to pT0N0 tumors, ranges from 15 to 29% in recent RCTs (57,60,61).
So the concept of organ preservation has emerged (62): local excision after NAT is a treatment for residual N0 tumors (subcomplete or complete response) but it could be also a macrobiopsy to confirm staging and to adapt final management (63). It is now recognized that there is a correlation between the tumor response and the nodal status response (63). For pT0 and pT1 tumors, Rullier and Vendrely underlined that the 7% risk of positive lymph nodes must be balanced with the 2% to 4% of operative morbidity in radical surgery for rectal cancer (63).
Oncologic results
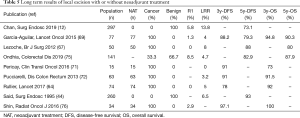
Long-term oncologic results need to be discussed for patients treated with neoadjuvant chemoradiotherapy followed by local excision. The recent results of the French multicenter phase III RCT GRECCAR 2, comparing local excision and TME after neoadjuvant chemoradiotherapy in good responders, showed a 3-year loco-regional recurrence rate of 5.4% and 3-year distant recurrence rate of 12.2% in the local excision group in the intention-to-treat population. There was no difference between the groups in term of local recurrence, distant recurrence, disease-free survival and overall survival (64). Five RCTs (64-68) and 6 non-randomized studies (69-74), aiming to analysis oncologic outcomes in patients treated with local excision after NAT, are listed in Table 4. Loco-regional recurrence rate must be carefully analyzed according to the methods of the studies (for example when the analysis is retrospective). But the GRECCAR 2 (64), the American ACOSOG Z6041 (69) or the Italian multicenter phase II trial (72) found interesting results for selected patients (good responders after NAT or initially small tumors). It is also important to notice that pCR rate for small tumors treated with NAT can reach 47%. Long-term results are presented in Table 5, in comparison to the few studies that report oncologic outcomes and long-term survival of local excision without any NAT, also in the case of benign tumors. Concerning studies evaluating long-term results after NAT, three-year disease-free survival varies from 78% to 91%, depending on the initial T and N of treated tumors, which is an acceptable oncologic outcome in these cases. In these papers, patients treated are good responders with a good oncologic prognosis. The other studies, with surgery first, usually include smaller tumors and present similar loco-regional recurrence rate and oncologic outcomes. The Table 5 results support the possibility to perform local transanal surgery for certain well-selected rectal cancers after NAT (12,44,64,67,69,71,72,75,76).
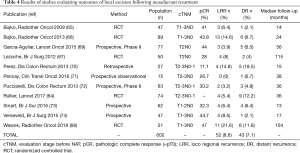
Full table
Full table
Morbidity
Overall peri-operative morbidity rate following local excision after NAT ranges from 8.8% to 58.4% in different series (67,69,74,76-78). Very few studies have focused on the comparison of morbi-mortality rates with or without NAT. Marks et al., in 2009, in a study with 62 patients, found an overall morbidity rate of 33% for the NAT group and 5.3% for the non-NAT group (P<0.05). The wound complication rate was also in favor of the non-NAT group (p0.015) (79). Two other studies reported results comparing morbidity rate with and without NAT: there was no significant difference (80,81).
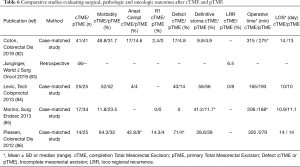
The main problem for rectal cancer treated with local excision is the existence of poor prognostic factors requiring a cTME. Indeed, a second surgery in the early postoperative period exposes the surgeon to surgical difficulties due to inflammation and fibrosis, with a risk of increased anastomotic failure but also poorer oncologic outcomes. In the GRECCAR 2 trial, there was no superiority of local excision over TME because the primary endpoint was a composite outcome including morbi-mortality. The outcomes in the local excision group were more complicated due to the specific morbidity rate after cTME, concerning 38% of the cases. Major morbidity rates (Dindo III-IV) was 46% in patients treated with cTME, and only 12% in the local excision group without cTME and 22% in the TME group (P=0.0031) (64). Four case-matched studies and one retrospective cohort study have compared surgical, pathologic and oncologic outcomes between local excision plus cTME and primary TME (pTME) (82-86). Results are summarized in Table 6. There is a trend in favor of pTME in terms of overall morbidity (for example anastomotic complications), quality of mesorectal excision, definitive stoma and operative time. The second option after local excision following NAT with poor pathologic prognostic factors is a strict surveillance with salvage TME when a local regrowth or recurrence is diagnosed. However, here again TME seems to be associated with more R1 resection and local re-recurrences (87).
Full table
Local excision after NAT for rectal cancer is clearly feasible with increasingly controlled outcomes. But a rigorous selection of cases and in particular according to the analysis of the tumor response is needed. The tools for interpreting this response (MRI, biomarkers) are constantly being improved and will allow the surgeon to adapt the surgical strategy.
Conclusion and future prospects
Management of rectal cancer is in permanent evolution (88). The intensification of the neoadjuvant treatment, called Total Neoadjuvant Therapy (TNT) which used induction or consolidation chemotherapy could increase tumor response (with a higher rate of pCR) (89). This high rate of tumoral response should help surgeon to adapt the strategy in order to decrease surgical morbidity and to increase quality of life. A French prospective multicentric phase III randomized trial is in progress investigating this strategy (GRECCAR 12): the aim is to increase organ preservation in rectal cancer adding induction Folfirinox before chemoradiotherapy (ClinicalTrials.gov Identifier: NCT02514278).
Another approach to preserve the rectum consists in local excision followed by adjuvant treatment such as chemoradiotherapy in case of poor pathologic features. It could avoid cTME and it seems to be feasible oncologically for selected tumors. The risk-benefit balance with the morbidity of cTME should be taken into account (90-92). The TESAR trial is investigating this strategy and will try to demonstrate the safety of adjuvant chemoradiotherapy after local excision (93).
In the near future, robotic devices could help local excision in selective difficult cases in particular within the limit of the TEM (94,95) but also with the help of a dedicated single port system (96).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.03.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parks AG, Stuart AE. The management of villous tumours of the large bowel. Br J Surg 1973;60:688-95. [Crossref] [PubMed]
- Buess G, Kipfmüller K, Hack D, et al. Technique of transanal endoscopic microsurgery. Surg Endosc 1988;2:71-5. [Crossref] [PubMed]
- Kraske P, Perry EG, Hinrichs B. A new translation of professor Dr P. Kraske’s Zur Exstirpation Hochsitzender Mastdarmkrebse. 1885. Aust N Z J Surg 1989;59:421-4. [PubMed]
- Meurette G. Description par Paul Kraske (1851-1930) en 1885 d’une voie d’abord périnéale élargie emportant les dernières pièces sacrées pour accéder aux tumeurs du haut rectum : celle qui deviendra « la voie de Kraske ». Côlon Rectum 2019;13:161-2. [Crossref]
- Mason AY. Transsphincteric approach to rectal lesions. Surg Annu 1977;9:171-94. [PubMed]
- Nivatvongs S, Wolff BG. Technique of per anal excision for carcinoma of the low rectum. World J Surg 1992;16:447-50. [Crossref] [PubMed]
- Buess G, Kipfmüller K, Ibald R, et al. Clinical results of transanal endoscopic microsurgery. Surg Endosc 1988;2:245-50. [Crossref] [PubMed]
- Kipfmüller K, Buess G, Naruhn M, et al. Training program for transanal endoscopic microsurgery. Surg Endosc 1988;2:24-7. [Crossref] [PubMed]
- Ramirez JM, Aguilella V, Arribas D, et al. Transanal full-thickness excision of rectal tumours: should the defect be sutured? a randomized controlled trial. Colorectal Dis 2002;4:51-5. [Crossref] [PubMed]
- Brown CJ, Hochman D, Raval MJ, et al. A multi-centre randomized controlled trial of open vs closed management of the rectal defect after transanal endoscopic microsurgery. Colorectal Dis 2019;21:1025-31. [Crossref] [PubMed]
- Brown C, Raval MJ, Phang PT, et al. The surgical defect after transanal endoscopic microsurgery: open versus closed management. Surg Endosc 2017;31:1078-82. [Crossref] [PubMed]
- Chan T, Karimuddin AA, Raval MJ, et al. Predictors of rectal adenoma recurrence following transanal endoscopic surgery: a retrospective cohort study. Surg Endosc 2020;34:3398-407. [Crossref] [PubMed]
- Hahnloser D, Cantero R, Salgado G, et al. Transanal minimal invasive surgery for rectal lesions: should the defect be closed? Colorectal Dis 2015;17:397-402. [Crossref] [PubMed]
- Noura S, Ohue M, Miyoshi N, et al. Significance of defect closure following transanal local full-thickness excision of rectal malignant tumors. Mol Clin Oncol 2016;5:449-54. [Crossref] [PubMed]
- Lee L, Althoff A, Edwards K, et al. Outcomes of Closed Versus Open Defects After Local Excision of Rectal Neoplasms: A Multi-institutional Matched Analysis. Dis Colon Rectum 2018;61:172-8. [Crossref] [PubMed]
- Menahem B, Alves A, Morello R, et al. Should the rectal defect be closed following transanal local excision of rectal tumors? A systematic review and meta-analysis. Tech Coloproctol 2017;21:929-36. [Crossref] [PubMed]
- Dafnis G, Påhlman L, Raab Y, et al. Transanal endoscopic microsurgery: clinical and functional results. Colorectal Dis 2004;6:336-42. [Crossref] [PubMed]
- Mege D, Petrucciani N, Maggiori L, et al. Peritoneal perforation is less a complication than an expected event during transanal endoscopic microsurgery: experience from 194 consecutive cases. Tech Coloproctol 2017;21:729-36. [Crossref] [PubMed]
- Saget A, Maggiori L, Petrucciani N, et al. Is there a limit to transanal endoscopic surgery? A comparative study between standard and technically challenging indications among 168 consecutive patients. Colorectal Dis 2015;17:O155-60. [Crossref] [PubMed]
- Kumar AS, Coralic J, Kelleher DC, et al. Complications of transanal endoscopic microsurgery are rare and minor: a single institution’s analysis and comparison to existing data. Dis Colon Rectum 2013;56:295-300. [Crossref] [PubMed]
- Lee L, Edwards K, Hunter IA, et al. Quality of Local Excision for Rectal Neoplasms Using Transanal Endoscopic Microsurgery Versus Transanal Minimally Invasive Surgery: A Multi-institutional Matched Analysis. Dis Colon Rectum 2017;60:928-35. [Crossref] [PubMed]
- Christoforidis D, Cho HM, Dixon MR, et al. Transanal endoscopic microsurgery versus conventional transanal excision for patients with early rectal cancer. Ann Surg 2009;249:776-82. [Crossref] [PubMed]
- de Graaf EJR, Burger JWA, van Ijsseldijk ALA, et al. Transanal endoscopic microsurgery is superior to transanal excision of rectal adenomas. Colorectal Dis 2011;13:762-7. [Crossref] [PubMed]
- Han J, Noh GT, Cheong C, et al. Transanal Endoscopic Operation Versus Conventional Transanal Excision for Rectal Tumors: Case-Matched Study with Propensity Score Matching. World J Surg 2017;41:2387-94. [Crossref] [PubMed]
- Moore JS, Cataldo PA, Osler T, et al. Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum 2008;51:1026-30; discussion 1030-1031. [Crossref] [PubMed]
- Clancy C, Burke JP, Albert MR, et al. Transanal endoscopic microsurgery versus standard transanal excision for the removal of rectal neoplasms: a systematic review and meta-analysis. Dis Colon Rectum 2015;58:254-61. [Crossref] [PubMed]
- Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 2010;24:2200-5. [Crossref] [PubMed]
- Mege D, Bridoux V, Maggiori L, et al. What is the best tool for transanal endoscopic microsurgery (TEM)? A case-matched study in 74 patients comparing a standard platform and a disposable material. Int J Colorectal Dis 2017;32:1041-5. [Crossref] [PubMed]
- Lee L, Kelly J, Nassif GJ, et al. Establishing the learning curve of transanal minimally invasive surgery for local excision of rectal neoplasms. Surg Endosc 2018;32:1368-76. [Crossref] [PubMed]
- Pigot F, Bouchard D, Mortaji M, et al. Local excision of large rectal villous adenomas: long-term results. Dis Colon Rectum 2003;46:1345-50. [Crossref] [PubMed]
- Bains L, Lal P, Vindal A, et al. Giant villous adenoma of rectum- what is the malignant potential and what is the optimal treatment? A case and review of literature. World J Surg Oncol 2019;17:109. [Crossref] [PubMed]
- Guerrieri M, Baldarelli M, Morino M, et al. Transanal endoscopic microsurgery in rectal adenomas: experience of six Italian centres. Dig Liver Dis 2006;38:202-7. [Crossref] [PubMed]
- Bretagnol F, Rullier E, George B, et al. Local therapy for rectal cancer: still controversial? Dis Colon Rectum 2007;50:523-33. [Crossref] [PubMed]
- Kikuchi R, Takano M, Takagi K, et al. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum 1995;38:1286-95. [Crossref] [PubMed]
- Verra M, Riente F, Arezzo A. Early rectal cancer treated by endoscopic submucosal dissection (ESD), endoscopic mucosal resection (EMR) or transanal endoscopic microsurgery (TEM). Ann Laparosc Endosc Surg 2018;3:67. [Crossref]
- Lakkis Z, Manceau G, Bridoux V, et al. Management of rectal cancer: the 2016 French guidelines. Colorectal Dis 2017;19:115-22. [Crossref] [PubMed]
- Beaton C, Twine CP, Williams GL, et al. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis 2013;15:788-97. [Crossref] [PubMed]
- Gérard JP, André T, Bibeau F, et al. Rectal cancer: French Intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis 2017;49:359-67. [Crossref] [PubMed]
- Bach SP, Hill J, Monson JRT, et al. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg 2009;96:280-90. [Crossref] [PubMed]
- Khoury W, Igov I, Issa N, et al. Transanal endoscopic microsurgery for upper rectal tumors. Surg Endosc 2014;28:2066-71. [Crossref] [PubMed]
- Baatrup G, Elbrønd H, Hesselfeldt P, et al. Rectal adenocarcinoma and transanal endoscopic microsurgery. Diagnostic challenges, indications and short term results in 142 consecutive patients. Int J Colorectal Dis 2007;22:1347-52. [Crossref] [PubMed]
- Maslekar S, Pillinger SH, Monson JRT. Transanal endoscopic microsurgery for carcinoma of the rectum. Surg Endosc 2007;21:97-102. [Crossref] [PubMed]
- Ramirez JM, Aguilella V, Gracia JA, et al. Local full-thickness excision as first line treatment for sessile rectal adenomas: long-term results. Ann Surg 2009;249:225-8. [Crossref] [PubMed]
- Said S, Stippel D. Transanal endoscopic microsurgery in large, sessile adenomas of the rectum. A 10-year experience. Surg Endosc 1995;9:1106-12. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Barendse RM, van den Broek FJC, van Schooten J, et al. Endoscopic mucosal resection vs transanal endoscopic microsurgery for the treatment of large rectal adenomas. Colorectal Dis 2012;14:e191-6. [Crossref] [PubMed]
- Cao Y, Liao C, Tan A, et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41:751-7. [Crossref] [PubMed]
- Park SU, Min YW, Shin JU, et al. Endoscopic submucosal dissection or transanal endoscopic microsurgery for nonpolypoid rectal high grade dysplasia and submucosa-invading rectal cancer. Endoscopy 2012;44:1031-6. [Crossref] [PubMed]
- Kawaguti FS, Nahas CSR, Marques CFS, et al. Endoscopic submucosal dissection versus transanal endoscopic microsurgery for the treatment of early rectal cancer. Surg Endosc 2014;28:1173-9. [Crossref] [PubMed]
- Arezzo A, Passera R, Saito Y, et al. Systematic review and meta-analysis of endoscopic submucosal dissection versus transanal endoscopic microsurgery for large noninvasive rectal lesions. Surg Endosc 2014;28:427-38. [Crossref] [PubMed]
- Morino M, Risio M, Bach S, et al. Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc 2015;29:755-73. [Crossref] [PubMed]
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Denost Q, Laurent C, Capdepont M, et al. Risk factors for fecal incontinence after intersphincteric resection for rectal cancer. Dis Colon Rectum 2011;54:963-8. [Crossref] [PubMed]
- Celentano V, Cohen R, Warusavitarne J, et al. Sexual dysfunction following rectal cancer surgery. Int J Colorectal Dis 2017;32:1523-30. [Crossref] [PubMed]
- Matthiessen P, Hallböök O, Rutegård J, et al. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 2007;246:207-14. [Crossref] [PubMed]
- Denost Q, Rouanet P, Faucheron JL, et al. To Drain or Not to Drain Infraperitoneal Anastomosis After Rectal Excision for Cancer: The GRECCAR 5 Randomized Trial. Ann Surg 2017;265:474-80. [Crossref] [PubMed]
- Lefevre JH, Mineur L, Kotti S, et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol 2016;34:3773-80. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- van Gijn W, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575-82. [Crossref] [PubMed]
- Akgun E, Caliskan C, Bozbiyik O, et al. Randomized clinical trial of short or long interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2018;105:1417-25. [Crossref] [PubMed]
- Terzi C, Bingul M, Arslan NC, et al. Randomized controlled trial of 8 weeks' vs 12 weeks' interval between neoadjuvant chemoradiotherapy and surgery for locally advanced rectal cancer. Colorectal Dis 2020;22:279-88. [Crossref] [PubMed]
- Rouanet P, Saint Aubert B, Fabre JM, et al. Conservative treatment for low rectal carcinoma by local excision with or without radiotherapy. Br J Surg 1993;80:1452-6. [Crossref] [PubMed]
- Rullier E, Vendrely V. Can mesorectal lymph node excision be avoided in rectal cancer surgery? Colorectal Dis 2011;13 Suppl 7:37-42. [Crossref] [PubMed]
- Rullier E, Rouanet P, Tuech JJ, et al. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet 2017;360:469-79. [Crossref] [PubMed]
- Bujko K, Richter P, Kołodziejczyk M, et al. Preoperative radiotherapy and local excision of rectal cancer with immediate radical re-operation for poor responders. Radiother Oncol 2009;92:195-201. [Crossref] [PubMed]
- Bujko K, Richter P, Smith FM, et al. Preoperative radiotherapy and local excision of rectal cancer with immediate radical re-operation for poor responders: a prospective multicentre study. Radiother Oncol 2013;106:198-205. [Crossref] [PubMed]
- Lezoche E, Baldarelli M, Lezoche G, et al. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg 2012;99:1211-8. [Crossref] [PubMed]
- Wawok P, Polkowski W, Richter P, et al. Preoperative radiotherapy and local excision of rectal cancer: Long-term results of a randomised study. Radiother Oncol 2018;127:396-403. [Crossref] [PubMed]
- Garcia-Aguilar J, Renfro LA, Chow OS, et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 2015;16:1537-46. [Crossref] [PubMed]
- Perez RO, Habr-Gama A, Lynn PB, et al. Transanal endoscopic microsurgery for residual rectal cancer (ypT0-2) following neoadjuvant chemoradiation therapy: another word of caution. Dis Colon Rectum 2013;56:6-13. [Crossref] [PubMed]
- Pericay C, Serra-Aracil X, Ocaña-Rojas J, et al. Further evidence for preoperative chemoradiotherapy and transanal endoscopic surgery (TEM) in T2-3s,N0,M0 rectal cancer. Clin Transl Oncol 2016;18:666-71. [Crossref] [PubMed]
- Pucciarelli S, De Paoli A, Guerrieri M, et al. Local excision after preoperative chemoradiotherapy for rectal cancer: results of a multicenter phase II clinical trial. Dis Colon Rectum 2013;56:1349-56. [Crossref] [PubMed]
- Smart CJ, Korsgen S, Hill J, et al. Multicentre study of short-course radiotherapy and transanal endoscopic microsurgery for early rectal cancer. Br J Surg 2016;103:1069-75. [Crossref] [PubMed]
- Verseveld M, de Graaf EJR, Verhoef C, et al. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg 2015;102:853-60. [Crossref] [PubMed]
- Ondhia M, Tamvakeras P, O’Toole P, et al. Transanal endoscopic microsurgery for rectal lesions in a specialist regional early rectal cancer centre: the Mersey experience. Colorectal Dis 2019;21:1164-74. [Crossref] [PubMed]
- Shin YS, Yoon YS, Lim SB, et al. Preoperative chemoradiotherapy followed by local excision in clinical T2N0 rectal cancer. Radiat Oncol J 2016;34:177-85. [Crossref] [PubMed]
- Guerrieri M, Gesuita R, Ghiselli R, et al. Treatment of rectal cancer by transanal endoscopic microsurgery: experience with 425 patients. World J Gastroenterol 2014;20:9556-63. [Crossref] [PubMed]
- Stipa F, Picchio M, Burza A, et al. Long-term outcome of local excision after preoperative chemoradiation for ypT0 rectal cancer. Dis Colon Rectum 2014;57:1245-52. [Crossref] [PubMed]
- Marks JH, Valsdottir EB, DeNittis A, et al. Transanal endoscopic microsurgery for the treatment of rectal cancer: comparison of wound complication rates with and without neoadjuvant radiation therapy. Surg Endosc 2009;23:1081-7. [Crossref] [PubMed]
- Coco C, Rizzo G, Mattana C, et al. Transanal endoscopic microsurgery after neoadjuvant radiochemotherapy for locally advanced extraperitoneal rectal cancer: short-term morbidity and functional outcome. Surg Endosc 2013;27:2860-7. [Crossref] [PubMed]
- Issa N, Murninkas A, Schmilovitz-Weiss H, et al. Transanal Endoscopic Microsurgery After Neoadjuvant Chemoradiotherapy for Rectal Cancer. J Laparoendosc Adv Surg Tech A 2015;25:617-24. [Crossref] [PubMed]
- Coton C, Lefevre JH, Debove C, et al. Does transanal local resection increase morbidity for subsequent total mesorectal excision for early rectal cancer? Colorectal Dis 2019;21:15-22. [Crossref] [PubMed]
- Junginger T, Goenner U, Hitzler M, et al. Local excision followed by early radical surgery in rectal cancer: long-term outcome. World J Surg Oncol 2019;17:168. [Crossref] [PubMed]
- Levic K, Bulut O, Hesselfeldt P, et al. The outcome of rectal cancer after early salvage TME following TEM compared with primary TME: a case-matched study. Tech Coloproctol 2013;17:397-403. [Crossref] [PubMed]
- Morino M, Allaix ME, Arolfo S, et al. Previous transanal endoscopic microsurgery for rectal cancer represents a risk factor for an increased abdominoperineal resection rate. Surg Endosc 2013;27:3315-21. [Crossref] [PubMed]
- Piessen G, Cabral C, Benoist S, et al. Previous transanal full-thickness excision increases the morbidity of radical resection for rectal cancer. Colorectal Dis 2012;14:445-52. [Crossref] [PubMed]
- Perez RO, Habr-Gama A, São Julião GP, et al. Transanal Endoscopic Microsurgery (TEM) Following Neoadjuvant Chemoradiation for Rectal Cancer: Outcomes of Salvage Resection for Local Recurrence. Ann Surg Oncol 2016;23:1143-8. [Crossref] [PubMed]
- Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol 2015;33:1797-808. [Crossref] [PubMed]
- Petrelli F, Trevisan F, Cabiddu M, et al. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann Surg 2020;271:440-8. [Crossref] [PubMed]
- Sasaki T, Ito Y, Ohue M, et al. Postoperative Chemoradiotherapy After Local Resection for High-Risk T1 to T2 Low Rectal Cancer: Results of a Single-Arm, Multi-Institutional, Phase II Clinical Trial. Dis Colon Rectum 2017;60:914-21. [Crossref] [PubMed]
- Borstlap WA, Coeymans TJ, Tanis PJ, et al. Meta-analysis of oncological outcomes after local excision of pT1-2 rectal cancer requiring adjuvant (chemo)radiotherapy or completion surgery. Br J Surg 2016;103:1105-16. [Crossref] [PubMed]
- Jeong JU, Nam TK, Kim HR, et al. Adjuvant chemoradiotherapy instead of revision radical resection after local excision for high-risk early rectal cancer. Radiat Oncol 2016;11:114. [Crossref] [PubMed]
- Borstlap WA, Tanis PJ, Koedam TWA, et al. A multi-centred randomised trial of radical surgery versus adjuvant chemoradiotherapy after local excision for early rectal cancer. BMC Cancer 2016;16:513. [Crossref] [PubMed]
- Warren CD, Hamilton AER, Stevenson ARL. Robotic transanal minimally invasive surgery (TAMIS) for local excision of rectal lesions with the da Vinci Xi (dVXi): technical considerations and video vignette. Tech Coloproctol 2018;22:529-33. [Crossref] [PubMed]
- Shuck RL, Larach SW, Atallah S. Robotic TAMIS for local excision of ultra-distal neoplasia. Tech Coloproctol 2019;23:395. [Crossref] [PubMed]
- Marks J, Ng S, Mak T. Robotic transanal surgery (RTAS) with utilization of a next-generation single-port system: a cadaveric feasibility study. Tech Coloproctol 2017;21:541-5. [Crossref] [PubMed]
Cite this article as: Carrier G, Rouanet P. Transanal Surgery for benign tumor or early rectal cancer: state of the art and future prospects of the surgeon. Ann Laparosc Endosc Surg 2021;6:6.


