The metabolic effects of surgery in type 2 diabetes
The prevalence of obesity is increasing all over the world. It has become one of the major public health problems due to its comorbidities and negative impact on the quality of life and life expectancy. According to the data of the World Health Organization, the worldwide incidence of obesity has tripled since 1975 (5%). In 2016, it was found that 1.9 billion adults were overweight (39%) and 650 million people (13%) were obese and the rate of those with a body mass index (BMI) of >35 was 4% and with a BMI of >40 was 1%. Obesity is the most well-known risk factor for type 2 diabetes mellitus (T2DM), and T2DM affects more than 400 million people, which is estimated to be around 650 million by 2040 (1,2). A study conducted in Turkey at 2012 found the conducted in Turkey found the prevalence of T2DM as 8% (3). Despite the drugs developed and lifestyle interventions, a significant portion of these patients are unable to achieve their treatment goals. Today, developing more effective treatment modalities for T2DM is on the list of top priorities.
The surgical methods used for obesity have been found to have positive effects on human metabolism beyond weight loss. The information obtained has shown that these methods are an important treatment alternative for T2DM patients. Because of these effects, the concept of “Metabolic Surgery” has been defined as “the manipulation of a normal organ or organ system to achieve a potential health gain” and used in addition to bariatric surgery (4,5). The current widely accepted indications for metabolic surgery are shown in Table 1. In addition to the classical indications for bariatric surgery, patients with uncontrolled diabetes and a BMI of 30–35 have been indicated to be a candidate for such surgeries. These indications are based on observation of the positive effects of alterations in the gastrointestinal tract following bariatric surgical procedures on glucose balance independent of weight loss (6). Both experimental and clinical studies in this respect have led to significant changes in the pathogenesis and treatment of T2DM. This article will summarize the relationship between glucose metabolism, obesity and T2DM, the effects of metabolic surgery on the gastrointestinal system (GIS) physiology, and the results of clinical studies.

Full table
Gastrointestinal physiology and glucose metabolism
Circulating glucose is primarily derived from complex carbohydrates taken with foods. It is also derived from precursors such as lactate, pyruvate, amino acids and glycerol via gluconeogenesis. Blood glucose levels are maintained in a narrow range by a number of mechanisms, including the small intestine.
Gastric emptying is regulated by the passage of gastric fluid to the duodenum by opening and closing of the pylorus in relation to blood glucose levels after food intake. Hyperglycemia slows gastric emptying, while hypoglycemia accelerates it. In addition, the duodenogastric feedback mechanism, vagovagal reflex, and GIS hormones are also involved in the regulation of gastric emptying. The enterocytes, which constitute the most important cell group of the small intestine epithelium, are the group of cells that have specific tasks and play a role in food absorption. When the amount of glucose in the intestinal lumen increases, the expression of the receptors, called sodium/glucose cotransporter 1 (SGLT1), which provide the absorption of glucose in the area from the duodenum to the ileum, increases. The increase in glucose absorption through this receptor increases the release of glucagon-like polypeptide (GLP-1) from L cells and gastric inhibitory polypeptide (GIP) from K cells. When glucose enters the enterocyte, it immediately passes to the interstitial space via basolateral glucose transporter (GLUT 2). This two-way transition is dependent on the glucose concentration in the enterocytes. An increase in glucose concentration in the enterocytes reduces hepatic gluconeogenesis. This mechanism is mainly regulated by the ventromedial hypothalamus where insulin receptors are present (7-9).
Glucose metabolism and gastrointestinal physiology are significantly affected by the GIS hormones secreted by enteroendocrine cells, at least 20 different subtypes of which have been found. The effects of these hormones on the normal intestinal tract and their alterations following metabolic surgery will be discussed in the following sections.
Insulin resistance (IR) and diabetes development
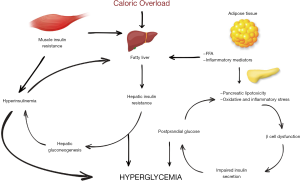
IR underlies obesity, T2DM, and cardiovascular diseases. IR is characterized by higher levels of glucose than its storage and use capacity in muscular tissue and an increase in gluconeogenesis in the liver. The majority of obese individuals with IR do not develop hyperglycemia since pancreatic beta (β) cells secrete more insulin to balance blood sugar. However, the continuation of excess glucose intake and the impairment of glucose tolerance in this process result in T2DM development. The studies have shown that hepatic IR is more important than muscle IR in the development of hyperglycemia. Excessive caloric load causes fat accumulation in the liver and fat accumulation causes the development of hepatic IR. The development of hepatic IR inhibits the suppressive effect of insulin on hepatic gluconeogenesis. Increased levels of glucose further increase basal insulin secretion. This results in a further increase in fat accumulation in the liver. This fatty liver and an excess of free fatty acids (FFA) disrupt acute insulin release by acting on β cells. Hyperglycemia causes more insulin secretion, increased hepatic lipogenesis, and lipotoxicity in pancreatic β cells. The development of T2DM from the fatty liver is a 2–3-year process. If not reversed, permanent damage occurs in β cells (10,11).
Another problem caused by overeating is that the stimuli from the intestinal system increase the fasting insulin level. Continuously elevated basal insulin level causes the muscle tissue to take up more glucose than it needs or can store. Glucose in the muscle tissue is first converted into pyruvate and subsequently to lactate and alanine. These metabolites are glucogenic substrates and increase the formation of hepatic glucose when they reach the liver (Cori cycle) (12).
Continuous hyperglycemia and increased FFAs from adipose tissue lead to low-grade persistent inflammation and oxidative stress in β cells. FFAs have an anti-insulin function that occurs during lipid metabolism. They increase hepatic gluconeogenesis and fat storage in muscles. Excessive release of FFA, adiponectins, and cytokines such as TNF-α and IL-6 that have destructive effects on β cells and increase apoptosis from adipose tissue into circulation significantly affects metabolism (13).
The development mechanisms of IR and T2DM are shown in Figure 1.
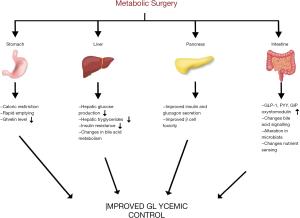
Gastrointestinal physiology, hormone changes and glucose metabolism after metabolic surgery
Some of the positive effects of metabolic surgery on blood glucose are undoubtedly caused by weight loss. A significant improvement has been observed in fasting glucose and first-phase insulin release of diabetic patients who underwent severe caloric restriction. However, the improvements in glucose metabolism, especially after Roux-en-Y gastric bypass (RYGB), are too positive that cannot be explained solely by weight loss. It starts in the earlier period, independent of weight loss (14).
In a normal gastrointestinal tract, food digestion and absorption are under the nervous system and hormonal effects. GIS hormones have significant effects on appetite, satiety, and food passage (cephalic, gastric and intestinal phase). These hormones are basically divided into two as incretins and anti-incretins in terms of their effects on insulin metabolism. After metabolic surgery, an increase has been observed in the levels of hormones with such effects, such as peptide YY (PYY), GLP-1, cholecystokinin, and GIP (15). After RYGB, gastric emptying accelerates for liquid foods, while it slows down for solid foods. Rapid gastric emptying and increased intestinal transit time are correlated with enteroglucagon and GLP-1 levels and weight loss. Because of partial resection of the antrum after sleeve gastrectomy (SG), the emptying of both solid and liquid foods increases. No change has been observed in the emptying of semi-solid food in SG cases where the antrum has been preserved. The rapid passage of food into the intestinal tract increases the release of GIS hormones (11,16). With the effect of hormones, weight loss increases and glucose balance improves. Both GLP-1 and insulin levels have been observed to decrease when food was delivered via a gastrostomy tube placed in the bypassed stomach after RYGB. When foods reach the ileum, a negative feedback occurs from distal to proximal, affecting jejunal motility, intestinal transit time, gastric emptying and pancreatic secretions. This is defined as the “ileal brake mechanism” and is mainly caused by GLP-1. It is not exactly known how this mechanism changes after metabolic surgery (17).
GLP-1 and GIP are two main incretin hormones that increase glucose-stimulated insulin secretion. There is not much research on GIP due to its release in very low amounts, glucagon release, and its less effect on eating behavior and appetite. On the other hand, GLP-1 is an anorexigenic and glucose metabolism regulator, which is located in the distal ileum and colon and released from intestinal L cells and the nucleus tractus solitarius in the brain. It has effects such as glucose-dependent insulin secretion, insulin synthesis, β-cell proliferation, cardioprotection, neuroprotection, satiety. It also reduces hepatic glucose uptake, β-cell apoptosis, glucagon secretion, and gastric emptying rate. Slower passage of food into the intestinal tract improves glucose balance. Intravenous administration of glucose affects GLP-1 levels very little and they are mainly affected by intestinal concentration. A significant increase is seen in its postprandial levels after metabolic surgery. The reason for this increase is explained by the passage of large amounts of food into the ileum due to the anatomical alteration (hindgut hypothesis) (18). Today, its agonists are used in the medical treatment of diabetes and obesity. GLP-1 exhibits its effects on appetite centrally by interacting with vagal afferent nerve fibers (7). After RYGB, GLP-1 levels have been found to be higher in those who succeeded in weight loss in the first year and lower in those who failed (19).
The alternative hypothesis is the foregut hypothesis. As a result of bypassing the upper intestinal tract, the decreased overstimulation with the interruption of the contact of foods with the duodenum and proximal jejunum probably causes the inactivation of anti-incretin factors. Thus, relatively excess incretin hormones increase the activity of insulin and produce positive effects on glucose metabolism. This hypothesis has been proposed with the observation of positive changes in the glucose metabolism of rats that underwent duodenal-jejunal bypass without creating any restriction in the stomach in experimental studies. However, it has not been clearly demonstrated (20).
Metabolic surgical interventions have different effects on insulin. Although fasting insulin levels have been shown to decrease after sleeve gastrectomy, the effects of RYGB surgery are more prominent in this regard. It has been shown that insulin sensitivity increases and the functions of β cell improve. These improvements are seen in the first weeks and are thought to be caused by GLP-1 increase (21).
Ghrelin, on the other hand, is a hormone that has an appetizing effect, suppresses insulin secretion and increases IR. Ghrelin levels increase with prolonged fasting and decrease postprandially. In general, its levels are reported to increase in those losing weight with calorie restriction and it is held responsible for the difficulty in long-term control of diet-induced weight loss. The long-term and short-term effects of metabolic surgery on ghrelin have not been completely clarified. After RYGB, a decrease has generally been observed in its levels. However, some studies have shown increased or normal levels. Although its levels decrease in the first months after surgeries such as RYGB and biliopancreatic diversion (BPD), an increase is observed in its levels in the first year. However, its levels decrease since the fundus where it is secreted most is removed after SG, which is kept responsible for the anti-diabetic effect of SG (22,23).
Changes in bile acid metabolism
Bile acids (BA) have important effects on glucose and lipid metabolism. They inhibit gluconeogenesis via membrane receptors in the ileum and liver (FXR and TGR5) and increase the activation of insulin signaling and glycogen synthesis. They stimulate the formation of postprandial fibroblast growth factor 19 (FGF19) in the ileum, increasing glycogen synthesis and reducing gluconeogenesis. Activation of these receptors increases GLP-1 release (24). While serum BA levels have been found to be increased after RYGB and SG, this effect could not be demonstrated after gastric band (GB). The most important cause of this increase is considered to be that BAs rapidly reach the distal ileum where FGF-1- and GLP-1-secreting cells are abundantly found. However, the results obtained are variable. There are studies showing a significant increase in serum SA levels within days, while there are also studies showing a significant increase in the first year after surgery (25). Some of the positive effects of metabolic surgical interventions causing changes in the GIS on glucose metabolism are considered to be caused by BA metabolism.
Microbiota
The bacterial structure of the intestinal tract is very complex and reaches very high numbers (1012/gram), especially in the distal ileum and colon. It is mainly composed of Bacteroidetes and Firmicutes groups. It has effects on BA metabolism, intestinal permeability, and inflammation modulation. Anaerobic bacteria convert primary BA into secondary BAs. Secondary BAs bind to TGR5 receptors, producing a positive effect on glucose balance and energy expenditure. The use of BA sequestrants in diabetic patients improves glycemic control. Intestinal bacteria ferment carbohydrates and turn them into short-chain FAs, a source for gluconeogenesis and lipogenesis. Short-chain FAs are also a factor for gut-brain signaling that affects the secretory and absorptive capacities of intestinal epithelial cells. There are not many studies on microbiota changes after metabolic surgery. An increase in Gammaproteobacteria and a decrease in Firmicutes have been found. Probiotic diet after RYGB increases weight loss in the early period (26). There is a need for further studies on microbiota change after metabolic surgery and its actual role.
Changes in the GIS after metabolic surgical procedures and their effects on glucose metabolism are shown in Figure 2.
Clinical study results
Numerous studies have been conducted on the metabolic effects of bariatric surgery. Almost all of the studies have shown a significant improvement in T2DM patients. The definitions of remission, improvement and worsening after metabolic surgery vary. While a fasting blood glucose (FBG) level of <1.10 g/L or an HbA1c of <6.5% for at least 1 year without treatment is generally considered as remission criterion, the American Diabetes Association (ADA) criteria for remission are more rigid and are considered as an HbA1c of <5.7% a glucose level of <5.6 mmol/dL. An HbA1c of <6.4% and a glucose level of <6.0 mmol/L are considered as partial remission (6). The criteria used when evaluating the results of the studies are important in the analysis of numbers (27,28).
In the meta-analysis of Buchwald et al. published in 2004, the rate of T2DM remission after bariatric surgery was found as 48% for GB, 72% for RYGB, 84% for BPD and 99% for duodenal switch. In this study, patients who achieved normal glucose levels without using medical treatment were considered as cured (29). In the Swedish Obese Subjects (SOS) study, an observational study, the outcomes of the surgical group and non-surgical group with indications were compared. During the 2-year follow-up, the rates of T2DM remission were 76% in the surgical group and 16% in the non-surgical group. In the 15-year follow-ups, the rates were 30.4% and 6.3%. In the 15-year follow-ups of patients with prediabetes at baseline, diabetes became prominent in 15.6% of patients in the surgical group and 54.5% of patients in the control group (30). In a meta-analysis of 6,373 patients analyzing twenty-one cohort studies, the two-year remission rate was 65%. The remission rate was 99% for BPD, 74% for RYGB, 61% for SG, and 33% for GB procedure (31). In another meta-analysis analyzing randomized controlled studies comparing metabolic surgery and medical treatment-follow-up, the efficacy of metabolic surgery in achieving remission was far superior to the medical group (RR: 22.1; P=0.002) (32). Hayoz et al. published a systematic review and meta-analysis of randomised controlled trials comparing the effects of RYGB with those of SG on metabolic outcome, with a special focus on glycaemic control. Based on their meta-analysis results, RYGB is more effective than SG in improving weight loss and short- and mid-term glycaemic and lipid metabolism control in patients with and without T2DM. They concluded that RYGB should be the first choice to treat patients with obesity and T2DM and/or dyslipidaemia (33). Despite some fictional differences in the studies conducted in this respect, the superiority of metabolic surgery is obvious.
In the Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) study, 150 T2DM patients with a BMI value ranging from 27 to 43 BMI were randomized to RYGB, SG, and intensive medical treatment. In the one-year follow-up, the remission rate was 12% in the medical treatment group, while it was 37% in the SG group and 42% in the RYGB group. In the 3-year follow-up, these rates were 5%, 24%, and 38%, respectively. The five-year results were obtained from 134 patients, and the remission rates were 5% in the medical group, 23% in the SG group, and 29% in the RYGB group. Similar results were also observed in terms of weight loss, quality of life and lipid levels (34-36). In the meta-analysis of Sheng et al. investigating T2DM remission, microvascular and macrovascular complications of bariatric surgery with at least five years of follow-up, the remission rate was found to have increased significantly (65% vs. 15.6%, RR: 5.90). There was a 79% decrease in microvascular complications (RR: 0.37) and a 48% decrease in macrovascular complications (RR: 0.52) and the mortality rate was 79% (RR: 0.21) lower than that of the medical treatment group (37). The study by Mingrone et al. compared medical treatment, RYGB and BPD in 60 patients with a BMI of >35 compared and found remission rates to be 75% for RYGB, 95% for BPD, and 0% for medical treatment in the two-year follow-up (38). In the meta-analysis of Panunzi et al. including 94,579 patients, no difference was found between the patients with a BMI of <35 and those with a BMI of >35 in terms of diabetes remission (71% vs. 72%) (39). Similar results were also obtained in the meta-analysis of Cummings et al. analyzing 11 randomized controlled studies (40). Despite the quite positive outcomes of metabolic surgery, the absence of adequate response in about 30% of patients suggests the need for good patient selection and information. There was no relationship between inadequate weight loss and response in the unresponsive patient group. Advanced age, long-term disease, polypharmacy and high HbA1c levels are important factors in failure of glycemic control after surgery (41,42).
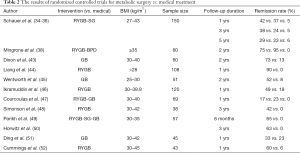
The results of randomized controlled studies are shown in Table 2 (34-36,38,43-52).
Full table
The choice of surgical method is another topic of discussion in this field. Metabolic activity is ranked as BPD > RYGB > SG > GB. For now, the most commonly performed metabolic surgery and the most widely accepted metabolic surgical intervention by the guidelines are RYGB, BPD and their modifications (53). The results of some new methods are also published.
New surgical interventions
In addition to interventions in the guidelines as the standard for metabolic surgical procedures, there are various studies and results on ileal interposition in combination with SG and SG + transit bipartition procedures. The first studies on ileal interposition were conducted by DePaula et al. on a group of 69 patients. In this study with a mean follow-up period of 21.7 months, the rate of patients with an HBA1c of <6 was reported to be 65.2% (54). In a study of 120 patients published by the same team in 2011, the remission rate was found as 84.2% (55). In a study of 30 patients published by Foschi et al. in 2019 comparing ileal interposition with standard medical treatment, the rate of 5-year complete T2DM remission was found as 68% (56). Transit bipartition was first proposed by Santoro in 2012 for metabolic syndrome with a remission rate of 86% (57). In a case series study by Yormaz et al. comparing ileal interposition, transit bipartition, and sleeve gastrectomy, the one-year remission was reported as 35.3%, 67.9%, and 54.7%, respectively (58). Although such surgical procedures are performed quite often in practice, both the number of cases in the published series is low and long-term results are insufficient. The initial remission rates obtained were close to RYGB outcomes and lower than BPD outcomes. However, the outcomes of such surgical interventions are still insufficient for routine use, and long-term outcomes remain lacking. Therefore, these procedures should be performed only within the study protocols.
Conclusions
Today, metabolic surgery is the only treatment modality that has the most positive and long-lasting effect on T2DM remission. Small intestines play a primary role in glucose balance with many mechanisms. These physiological effects are enhanced by some changes made in bariatric surgery. Metabolic surgical methods affect morphology, endocrine functions and digestive system physiology. Although a significant portion of its effects is related to weight loss, rapid changes in GIS hormones are particularly responsible for early metabolic effects. They show their effects with increased incretin hormone levels, decreased anti-incretin hormone levels, central effect through the vagus, change in bile acid metabolism, GIS microbiota change.
The clinical studies have confirmed these effects and therefore the indications for metabolic surgery have expanded. The standard recommended methods for metabolic surgery include SG, RYGB and BPD and their modifications. When and what method to use for which patient will be clarified by future studies. There is a need for studies with larger sample size and long-term results for the new methods to be used in daily practice.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mehmet Mahir Ozmen) for the series “Bariatric and Metabolic Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-19-248). The series “Bariatric and Metabolic Surgery” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2015 obesity collaborators, Afsinh A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13-27.
- Ng M, Fleming T, Robinson M, et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults During 1980-2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766-81. [Crossref] [PubMed]
- Satman I, Imamoğlu S, Yilmaz C, et al. Diabetes in Turkey and World. Turk J Endocrinol Metab 2012;16:1-56.
- Rubino F, Kaplan LM, Schauer PR, et al. The Diabetes Surgery Summit consensus conference: Recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg 2010;251:399-405. [Crossref] [PubMed]
- Rubino F, Cummings DE. Surgery: the coming of age of metabolic surgery. Nat Rev Endocrinol 2012;8:702-4. [Crossref] [PubMed]
- American Diabetes Association. 7. Approaches to glycemic treatments. Diabetes Care 2015;38:S41-S48. [Crossref] [PubMed]
- Quercia I, Dutia R, Kotler DP, et al. Gastrointestinal changes after bariatric surgery. Diabetes Metab 2014;40:87-94. [Crossref] [PubMed]
- Kamvissi-Lorenz V, Raffaelli M, et al. Role of the gut on glucose homeostasis: Lesson learned from metabolic surgery. Curr Atheroscler Rep 2017;19:9. [Crossref] [PubMed]
- Levin BE, Magnan C, Dunn-Meynell A, et al. Metabolic sensing and the brain: who,what,where, and how? Endocrinology 2011;152:2552-7. [Crossref] [PubMed]
- Cătoi AF, Parvu A, Mureşan A, et al. Metabolic Mechanisms in Obesity and Type 2 Diabetes: Insights From Bariatric/Metabolic Surgery. Obes Facts 2015;8:350-63. [Crossref] [PubMed]
- Genser L, Mariolo JRC, Castagneto-Gissey L, et al. Obesity, type 2 diabetes, and the metabolic syndrome. Surg Clin North Am 2016;96:681-701. [Crossref] [PubMed]
- Tappy L, Acheson K, Normand S, et al. Effects of glucose and amino acid infusion on glucose turn over in insulin-resistant obese and type II diabetic patients. Metabolism 1994;43:428-34. [Crossref] [PubMed]
- Sinclair P, Docherty N, le Roux CW. Metabolic effects of bariatric surgery. Clin Chem 2018;64:72-81. [Crossref] [PubMed]
- Özmen MM. Metabolic effects of bariatric surgery on the type 2 diabetes mellitus. Laparosc Endosc Surg Sci 2016;23:147-54.
- Sweeney TE, Morton JM. Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol 2014;28:727-40. [Crossref] [PubMed]
- Meek CL, Lewis HB, Reimann F, et al. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 2016;77:28-37. [Crossref] [PubMed]
- Barreto SG, Soenen S, Chisholm J, et al. Does the ileal brake mechanism contribute to sustained weight loss after bariatric surgery. ANZ J Surg 2018;88:20-5. [Crossref] [PubMed]
- Azim S, Kashyap SR. Bariatric surgery. Pathophysiology and outcomes. Endocrinol Metab Clin North Am 2016;45:905-21. [Crossref] [PubMed]
- Laferrère B. Effect of gastric bypass surgery on incretins. Diabetes Metab 2009;35:513-7. [Crossref] [PubMed]
- Rubino F, R’bibo S, del Genio F, et al. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol 2010;6:102-9. [Crossref] [PubMed]
- Holst JJ, Madsbad S, Bojsen-Moller KN, et al. Mechanisms in Bariatric Surgery: Gut Hormones, Diabetes Resolution, and Weight Loss. Surg Obes Relat Dis 2018;14:708-14. [Crossref] [PubMed]
- Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002;346:1623-30. [Crossref] [PubMed]
- McCarty TR, Jirapinyo P, Thompson CC. Effect of Sleeve Gastrectomy on Ghrelin, GLP-1, PYY, and GIP Gut Hormones: A Systematic Review and Meta-analysis. Ann Surg 2020;272:72-80. [Crossref] [PubMed]
- Penney NC, Kinross J, Newton RC, et al. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes (Lond) 2015;39:1565-74. [Crossref] [PubMed]
- El Khoury L, Chouillard E, Chahine E, et al. Metabolic surgery and diabesity: a systematic review. Obes Surg 2018;28:2069-77. [Crossref] [PubMed]
- Kootte RS, Vrieze A, Holleman F, et al. The therapeutic potential of manipulating gut microbiata in obesity and type 2 diabetes mellitus. Diabetes Obes Metab 2012;14:112-20. [Crossref] [PubMed]
- Fried M, Yumuk V, Oppert JM, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg 2014;24:42-55. [Crossref] [PubMed]
- Pareek M, Schauer PR, Kaplan LM, et al. Metabolic surgery: Weight loss, diabetes, and beyond. J Am Coll Cardiol 2018;71:670-87. [Crossref] [PubMed]
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-37. [Crossref] [PubMed]
- Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273:219-34. [Crossref] [PubMed]
- Yu J, Zhou X, Li L, et al. The long term effects of bariatric surgery for type 2 diabetes: systematic review and meta-analysis of randomized and non randomized evidence. Obes Surg 2015;25:143-58. [Crossref] [PubMed]
- Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity:a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347:f5934. [Crossref] [PubMed]
- Hayoz C, Hermann T, Raptis DA, et al. Comparison of metabolic outcomes in patients undergoing laparoscopic roux-en-Y gastric bypass versus sleeve gastrectomy - a systematic review and meta-analysis of randomised controlled trials. Swiss Med Wkly 2018;148:w14633. [PubMed]
- Kashyap SR, Bhatt DL, Schauer PR, et al. Bariatric surgery vs advanced practice medical management in the treatment of type 2 diabetes mellitus: rationale and design of the Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently Trial (STAMPEDE). Diabetes Obes Metab 2010;12:452-54. [Crossref] [PubMed]
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002-13. [Crossref] [PubMed]
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-Year outcomes. N Engl J Med 2017;376:641-51. [Crossref] [PubMed]
- Sheng B, Truong K, Spitler H, et al. The Long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: A systematic review and meta-analysis. Obes Surg 2017;27:2724-32. [Crossref] [PubMed]
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5-year follow-up of an open-label, single-centre randomised controlled trial. Lancet 2015;386:964-73. [Crossref] [PubMed]
- Panunzi S, De Gaetano A, Carnicelli A, et al. Predictors of remission of diabetes mellitus in severely obese individuals undergoing bariatric surgery: do BMI or procedure choice matter? A meta-analysis. Ann Surg 2015;261:459-67. [Crossref] [PubMed]
- Cummings DE, Rubino F. Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia 2018;61:257-64. [Crossref] [PubMed]
- Park JY. Prediction of type 2 diabetes remission after bariatric or metabolic surgery. J Obes Metab Syndr 2018;27:213-22. [Crossref] [PubMed]
- de Oliveira VL, Martins GP, Mottin CC, et al. Predictors of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass in severely obese patients. Obes Surg 2018;28:195-203. [Crossref] [PubMed]
- Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316-23. [Crossref] [PubMed]
- Liang H, Guan W, Yang Y, et al. Roux-en-Y Gastric Bypass for Chinese Type 2 Diabetes Mellitus Patients With a BMI < 28 kg/m(2): A Multi-Institutional Study. J Biomed Res 2015;29:112-7. [PubMed]
- Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomized controlled trial. Lancet Diabetes Endocrinol 2014;2:545-52. [Crossref] [PubMed]
- Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomized, controlled trial. Lancet Diabetes Endocrinol 2015;3:413-22. [Crossref] [PubMed]
- Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: A randomized clinical trial. JAMA Surg 2015;150:931-40. [Crossref] [PubMed]
- Simonson DC, Halperin F, Foster K, et al. Clinical and patient-centered outcomes in obese patients with type 2 diabetes 3 years after randomization to roux-en-Y gastric bypass surgery versus intensive lifestyle management: The SLIMM-T2D study. Diabetes Care 2018;41:670-9. [Crossref] [PubMed]
- Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do not meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg 2014;260:617-22; discussion 622-4. [Crossref] [PubMed]
- Horwitz D, Saunders JK, Ude-Welcome A, et al. Three-year follow-up comparing metabolic surgery versus medical weight management in patients with type 2 diabetes and BMI 30-35. The role of sRAGE biomarker as predictor of satisfactory outcomes. Surg Obes Relat Dis 2016;12:1337-41. [Crossref] [PubMed]
- Ding SA, Simonson DC, Wewalka M, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: A randomized clinical trial. J Clin Endocrinol Metab 2015;100:2546-56. [Crossref] [PubMed]
- Cummings DE, Aterburn DE, Westbrook EO, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomized controlled trial. Diabetologia 2016;59:945-53. [Crossref] [PubMed]
- Brito JP, Montori VM, Davis AM. Metabolic surgery in the treatment algorithm for type 2 diabetes: A joint statement by international diabetes organizations. JAMA 2017;317:635-6. [Crossref] [PubMed]
- DePaula AL, Macedon AL, Mota BR, et al. Laparoscopic ileal interposition associated to a diverted sleeve gastrectomy is an effective operation fort he treatment of type 2 diabetes mellitus patients with BMI 21-29. Surg Endosc 2009;23:1313-20. [Crossref] [PubMed]
- DePaula AL, Stival AR, Halpern A, et al. Surgical treatment of morbid obesity:mid-term outcomes of the laparoscopic ileal interposition associated to a sleeve gastrectomy in 120 patients. Obes Surg 2011;21:668-75. [Crossref] [PubMed]
- Foschi D, Sorrentino L, Tubazio I, et al. Ileal Interposition Coupled With Duodenal Diverted Sleeve Gastrectomy Versus Standard Medical Treatment in Type 2 Diabetes Mellitus Obese Patients: Long-Term Results of a Case-Control Study. Surg Endosc 2019;33:1553-63. [Crossref] [PubMed]
- Santoro S, Castro LC, Velhote MC, et al. Sleeve gastrectomy with transit bipartition: a potent intervention for metabolic syndrome and obesity. Ann Surg 2012;256:104-10. [Crossref] [PubMed]
- Yormaz S, Yılmaz H, Ece I, et al. Laparoscopic ileal interposition with diverted sleeve gastrectomy versus laparoscopic transit bipartition with sleeve gastrectomy for better glycemic outcomes in T2DM patients. Obes Surg 2018;28:77-86. [Crossref] [PubMed]
Cite this article as: Özgüç H. The metabolic effects of surgery in type 2 diabetes. Ann Laparosc Endosc Surg 2021;6:29.