Flexible endoscopy and hiatal hernias
Flexible endoscopy in upper gastrointestinal surgery
Flexible endoscopic evaluation of the esophagus and stomach is a vital element of upper gastrointestinal surgery. Upper endoscopy serves as a “physical examination” of the esophagus and stomach. As such, it should be performed on all patients in preparation for, and those undergoing upper gastrointestinal surgery, ideally by the operating surgeon. There is no substitute for the first-hand visualization of the pharyngeal, laryngeal, esophageal, gastric and duodenal mucosa, and anatomic pathology in the preoperative workup of the patient. Endoscopy allows assessment of anatomic correlates such as the presence of a stricture, esophagitis, Barrett’s esophagus, a large hiatal hernia or intrathoracic stomach, esophageal dilation, or anatomical problems with prior upper gastrointestinal surgery among various other variables that may influence operative decisions (1). Biopsy evaluation of the distal esophagus, cardia, and stomach is also an important part of the assessment of upper gastrointestinal pathology (1).
Flexible endoscopy is vitally important in aiding with quality control intra-operatively. Issues such as adequacy of intraoperative esophageal length, snugness of hiatal repair, symmetry of the antireflux procedure, ruling out leaks, the management of intraoperative complications etc., are managed using flexible endoscopy. Flexible endoscopy becomes even more important in reoperative surgery. Combined laparoscopy and flexible endoscopy are often required to delineate postoperative anatomy in a hostile operative field.
Finally, flexible endoscopy is indispensable in the management of both early and late complications resulting from upper gastrointestinal surgery, especially hiatal hernia repairs and antireflux surgery. Esophageal dilations for postoperative dysphagia, endoscopic diversion with esophageal stents, or endoscopic drainage of perioperative fluid collections are some of the roles that flexible endoscopy offers in the immediate postoperative period. Late postoperative complications such as stricture, or herniated or slipped fundoplication are also primarily diagnosed using flexible endoscopy. Hence flexible endoscopy performs an irreplaceable role in all three phases of the surgical treatment of hiatal hernias. Every hiatal hernia surgeon should incorporate the use of flexible endoscopy in their practice. This chapter primarily focuses on the use of flexible endoscopy in the preoperative phase of hiatal hernia management.
Endoscopic evaluation of the hiatus
The locations of three landmarks are routinely obtained during antegrade upper endoscopy. In relation to the front incisors these include: the squamocolumnar junction, the gastroesophageal junction, and the diaphragmatic crura. The squamocolumnar junction can be easily identified as the location where the velvet and darker rose-colored columnar epithelium changes to the lighter squamous epithelium. In instances when this junction is not clear, narrow band imaging (NBI), a helpful feature standard on all modern endoscopic systems, can be utilized to distinguish columnar from squamous mucosa. Particular attention should be paid in order to detect any tongues, islands, or circumferential segments of columnar-lined esophagus in the distal esophagus. Next, the gastroesophageal junction is identified. This is the location where gastric rugal folds meet the tubular esophagus. Under normal circumstances, the gastroesophageal junction is aligned with the squamocolumnar junction. Finally, the diaphragmatic crura can be identified by having the patient sniff (2).
The distance in centimeters between the proximal extent of the squamo-columnar junction and the rugal folds gives the extent of the columnar lined esophagus. The standardized Prague classification should be used in the reporting of the columnar lined esophagus (Marginal and Circumferential extent). The distance between the proximal extent of the rugal folds and the diaphragmatic pinch gives the axial height of the hiatal hernia. The diagnosis of a hiatal hernia is made when separation of the gastroesophageal junction from the crura is visualized. By definition, a hiatal hernia is present when at least 2 cm of the top of the rugal folds has migrated above the pinch of the diaphragmatic crura. When a hiatal hernia is diagnosed, particular care should be taken to exclude Cameron ulcers or gastritis within the herniated stomach (2).
Most of the important information in the endoscopic evaluation of hiatal hernias, however, is obtained in retroflexion view. Retroflexion of the endoscope in the stomach enables the clinician to identify abnormalities of the cardia or gastroesophageal junction. Furthermore, the retroflexion view provides a complementary assessment regarding the competency of the gastroesophageal barrier. Hill et al. have graded the appearance of the gastroesophageal valve from I to IV according to the degree of unfolding or deterioration of the normal valve architecture (3). This commonly used grading system allows endoscopists to maintain uniformity in their reporting (2). The Hill grade evaluation of the gastrointestinal junction is performed after maximal insufflation of the stomach.
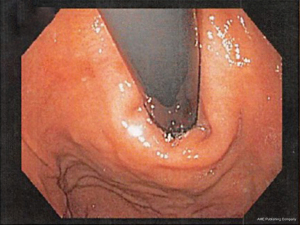
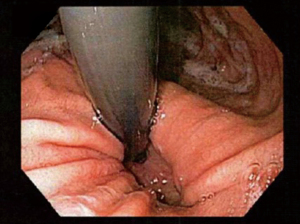
A Hill grade I valve is defined by the presence of a prominent fold of tissue closely approximated to the shaft of the endoscope and extending 3–4 cm along the lesser curvature at the entrance of the esophagus into the stomach (see Figure 1).
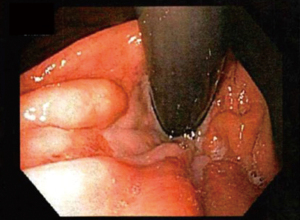
In a Hill grade II gastroesophageal valve, the fold of tissue is less prominent, and there are occasional periods of opening and rapid closing around the endoscope with respiration (see Figure 2).
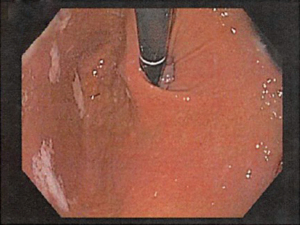
In a Hill grade III gastroesophageal valve, there is no prominent fold at the entrance of the esophagus into the stomach, and the endoscope is not tightly gripped by the tissues (see Figure 3).
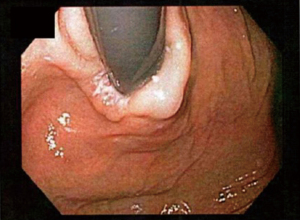
In a Hill grade IV gastroesophageal valve, the patient has a hiatal hernia and essentially no fold where the lumen of the endoscope is gaping open, allowing the squamous epithelium to be viewed from below (see Figure 4).
When a normal looking valve is encountered on maximal insufflation an attempt should be made to pass the retroflexed tip of the flexible scope along the lesser curve of the stomach back through the gastrointestinal junction. This maneuver further stresses the lower esophageal sphincter in addition to maximal insufflation and might uncover a small sliding hiatal hernia.
On slow withdrawal of the endoscope, the esophagus should be again examined and biopsy samples should be obtained. Another important landmark that should be located is the cricopharyngeus. The larynx and vocal cords are visualized. The endoscopist should note any inflammation of the larynx, which can occur secondary to acid reflux. Finally, vocal cord movement should be recorded, both as a reference for subsequent surgery and as an assessment of the patient’s ability to protect the airway (2).
Classification of hiatal hernias
Hiatal hernias are classified anatomically into four types (I to IV), depending on the relationship of the gastro-esophageal junction and the gastric fundus to the diaphragmatic hiatus.
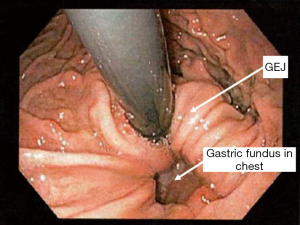
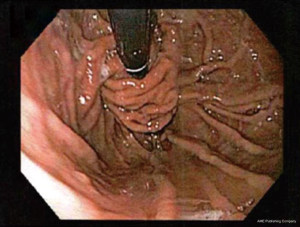
Type I hiatal hernia, also called a sliding hiatal hernia, is the most common, constituting 90% of all hiatal hernias. A type I hernia is present when the gastro-esophageal junction migrates cephalad into the posterior mediastinum. This occurs because of laxity of the phrenoesophageal membrane, a continuation of the endoabdominal peritoneum that reflects onto the esophagus at the hiatus. Although these hernias do slide back and forth through the hiatus, they are called sliding hernias because the stomach is part of the wall of the hernial sac. Thus, they are analogous with sliding inguinal hernias, which typically involve partially retroperitoneal organs (4). The presence of a type I sliding hiatal hernia alone does not constitute an indication for operative repair. In fact, many patients with small type I hiatal hernias do not have symptoms and do not require treatment (5). During flexible endoscopy, the gastro-esophageal junction is seen at the roof of the herniated stomach. Sliding hernias can grow to become quite large and may result in medically refractory reflux symptoms, warranting surgical intervention (see Figures 5,6).
Hiatal hernia types II to IV represent forms of paraesophageal herniation. A type II hernia, also known as a rolling hernia, occurs when the gastro-esophageal junction is anchored in the abdomen, and the gastric fundus migrates into the mediastinum through the hiatal defect. True paraesophageal (type II) hernias are uncommon, constituting about 3% of all hiatal hernias (4) (see Figure 7).
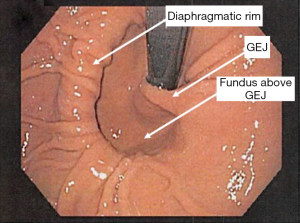
Type III hiatal hernias involve elements of both type I and II hernias and represent the majority of paraesophageal hernias presenting for surgical repair. Most often, enlargement of a type I hernia defect allows cephalad migration of the stomach in response to the transdiaphragmatic pressure gradient. A true hernia sac is present with fundic herniation and proximal migration of the gastroesophageal junction into the thorax. This type of hernia is associated with laxity of the elements that retain normal gastric position, and the natural history is to progress to complete gastric herniation with the appearance of an upside-down intrathoracic stomach on contrast radiography. On flexible endoscopy, the herniated fundus appears in a more cephalad location as compared with the gastroesophageal junction (see Figure 8).
Large hernia defects that contain other viscera or abdominal organs (e.g., colon, spleen, pancreas, or small bowel) are referred to as type IV hiatal hernias, but are best thought of as a subgroup of type III (4). Type IV hernias are diagnosed via an imaging modality such as computed tomography. On endoscopic examination they look similar to type I, II or III depending on the location of the gastroesophageal junction.
This classification system can lead to confusion as some hernias may appear to be either a sliding or paraesophageal hiatal hernia depending on patient position. Thus, the term “giant paraesophageal hernia” has been used to describe the large hernias in which at least half the stomach is located within the thorax on contrast radiography, the hernia measures at least 6 cm in length on preoperative endoscopy, or a distance between the crura of at least 5 cm is noted on intraoperative inspection. Giant hiatal hernias are repaired using the same principles required for all paraesophageal hernias, but the large hernia sac and propensity for esophageal shortening make these cases challenging (4).
An extremely rare form of diaphragmatic hernia is the parahiatal hernia. This type of hernia, with unknown incidence, has only been reported in a handful of case reports around the world. Parahiatal hernias are so rare that they are often misdiagnosed as paraesophageal hernias and are very difficult to diagnose preoperatively. The etiology of parahiatal hernias is believed to be either primary or congenital, caused by an incomplete obliteration of the embryonic pleuroperitoneal canal resulting in a persistent pneumoenteric defect, typically seen on the left side. More commonly, however, are the secondary or acquired parahiatal hernias. These hernias can arise from excessive crural manipulation during esophagectomies or antireflux procedures. On endoscopy, retroflexion will reveal a defect near the hiatus involving the gastric cardia which can be difficult to differentiate from a paraesophageal hernia or a gastric diverticulum near the gastroesophageal junction (see Figure 9).
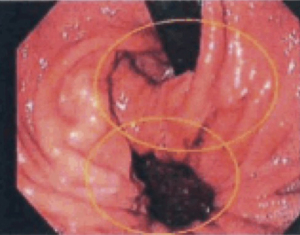
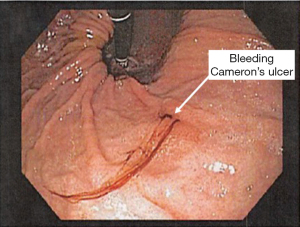
Large hiatal hernias can be associated with mucosal ulcerations from ischemia resulting from friction between the gastric wall and the diaphragmatic hiatus. These lesions are called Cameron’s ulcers and can commonly result in iron deficiency anemia from chronic gastrointestinal blood loss (see Figure 10). Rarely, they can present with life threatening bleeding. Cameron’s ulcers can be transient in nature and may or may not be seen on flexible endoscopy, especially in patients on chronic acid suppression therapy. Surgical repair of a large hiatal hernia should be considered in patients with undiagnosed iron deficiency despite whether or not these lesions are seen on endoscopy.
Recurrent hiatal hernia
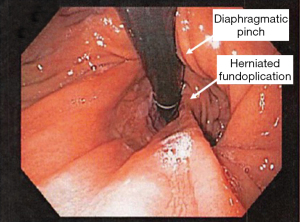
A hiatal hernia can recur after a prior surgical repair. These patients can present with typical reflux symptoms, esophageal outflow obstruction symptoms, chest or abdominal pain, or rarely extra-esophageal symptoms. Flexible endoscopy is an important tool in the evaluation of these patients. Careful endoscopic assessment of the gastro-esophageal junction is important so that a small herniation of the fundoplication is not missed. A redundant posterior portion of the fundoplication increases the risk of this complication (see Figures 11-14).
Acute gastric volvulus and strangulation
A relatively rare occurrence, acute gastric volvulus is a clinical emergency. Patients present with sudden onset of chest or epigastric pain associated with retching without the production of emesis. The development of fever, tachycardia, and leukocytosis suggests gastric strangulation and impending perforation. Gastric volvulus is necessary but not sufficient for gastric ischemia to develop. More often, patients present with subacute or chronic recurrent gastric volvulus, which causes gastroesophageal obstructive symptoms in the absence of gastric ischemia (5).
The anatomic classification of gastric volvulus is based on the axis of rotation. Organoaxial volvulus is the most common type, and it accounts for almost all cases of acute gastric volvulus.
This involves rotation of the stomach around the anatomic (longitudinal) axis, represented as a line drawn from the cardia to the pylorus, frequently resulting in gastric strangulation. In mesoentericoaxial volvulus, the antrum of the stomach rotates anteriorly and superiorly around a transverse axis that extends from the mid-lesser curvature to the mid-greater curvature. The rotation is typically incomplete and results in intermittent gastric obstruction, rather than acute strangulation (4).
Initial management of acute gastric volvulus should include placement of a nasogastric tube for gastric decompression. If bedside placement of a nasogastric tube is not possible, esophagoscopy can facilitate gastric decompression and nasogastric tube placement. Flexible endoscopy also commonly aids in the reduction of the volvulus. Additionally, endoscopy allows assessment of the gastric mucosa; if gastric ischemia is present, emergent operation is indicated (5). If the situation can be temporized either with a nasogastric tube or flexible endoscopy, a semi-urgent approach with laparoscopy performed by a more experienced surgeon may result in better short and long-term outcomes in terms of recurrence rates.
Conclusions
Flexible endoscopy is an integral part of the evaluation of a patient with a hiatal hernia. It serves as a vital tool in evaluating foregut anatomy. Flexible endoscopy should be utilized by all surgeons to aid in the preoperative workup, intraoperative quality control, and in the postoperative care of their patients, in addition to aiding in the management of complications and side-effects.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee L. Swanstrom and Steven G. Leeds) for the series “Hiatal Hernia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-48). The series “Hiatal Hernia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Niebisch S, Peters JH. Benign disorders of the esophagus and stomach: Anatomic and physiologic testing of esophageal function. In: Swanstrom LL, Soper NJ, Leonard ME, et al. editors. Mastery of endoscopic and laparoscopic surgery. Philadelphia: Lippincott Williams & Wilkins, 2014:75-90.
- Oh DS, DeMeester SR. Esophageal anatomy and physiology and gastroesophageal reflux disease. In: Mulholland MW, Lillemoe KD, Doherty GM, et al. editors. Greenfield's surgery: scientific principles and practice. Philadelphia: Wolters Kluwer, 2017:2006.
- Oberg S, Peters JH, DeMeester TR, et al. Endoscopic grading of the gastroesophageal valve in patients with symptoms of gastroesophageal reflux disease (GERD). Surg Endosc 1999;13:1184-8. [Crossref] [PubMed]
- Ricketts RR, Loukas M, Skandalakis LJ, et al. Surgical anatomy of the diaphragm. In: Fischer JE, Jones DB, Pomposelli FB, et al. editors. Fischer's Mastery of surgery. Philadelphia: Lippincott Williams & Wilkins, 2012:718-89.
- Yates RB, Oelschlager BK, Pellegrini CA. Gastroesophageal reflux disease and hiatal hernia. In: Townsend CM, Evers BM, Beauchamp RD, et al. editors. Sabiston textbook of surgery: the biological basis of modern surgical practice. Philadelphia: Elsevier, 2017:1043-64.
Cite this article as: Hennig A, Kurian AA. Flexible endoscopy and hiatal hernias. Ann Laparosc Endosc Surg 2021;6:45.