
Indication and results of endoscopic submucosal dissection for right located lateral spreading tumors
Introduction
Endoscopic submucosal dissection (ESD) is a technique used for en bloc resection and curative resection of larger mucosal cancers or slightly invasive submucosal cancers in gastrointestinal tract (1). ESD has developed in Japan in 1990s to overcome the limitation connected with the conventional endoscopic mucosal resection (EMR) and it represents a less invasive alternative to surgery for gastrointestinal lesions with minimally invasive cancer. At first, ESD was performed in the upper gastrointestinal tract to remove localized gastric tumors, then it was also applied to resect lesions in the colorectum. Although colorectal ESD is technically difficult and it is associated with a high risk of procedure-related adverse advents, like perforation and bleeding, this endoscopic technique has progressively become more safe and effective and complications are gradually improved with experience (2).
Colorectal ESD: indications
ESD is performed in colorectal lesions, which have a low incidence of lymph node metastasis, where standard techniques of endoscopic resection, such as polypectomy or EMR, are difficult to perform (1).
According to the guidelines of the Japanese Society for Cancer of the Colon and the Rectum (JSCCR), colorectal lesions removed endoscopically can be considered with low risk of lymph node metastasis when all the following criteria are respected: R0 margins, submucosal invasion depth <1,000 m, low grade (1 or 2) tumor differentiation, absence of lymphatic or vascular invasion, grade 1 tumor budding.
If one or more of these criteria is not respected, the patient should be referred for surgical treatment (3,4).
The macroscopical morphology (MP) of colorectal lesions can be predictive of the risk of submucosal invasion and may orientate towards the most appropriate treatment (5). It is commonly used the Paris Classification to evaluate the MP of the lesions, which divides them in polypoid lesions, that have an arbitrary height greater than 2.5 millimeters, and non-polypoid lesions, with a height less than 2.5 millimeters. When a lesion is flat or slightly protruding and its diameter is greater than 10 mm, it’s defined lateral spreading tumor (LST) (6). LST can be classified as granular (LST-G) and non granular (LST-NG) (Figure 1): LST-G is characterized by multiple nodules and it may present a homogeneous pattern (LST-GH), with a risk of submucosal invasion <2%, or mixed-size nodules (LST-G-NM), where the risk of submucosal invasion is increased up to 30% for lesions greater than 30 mm.
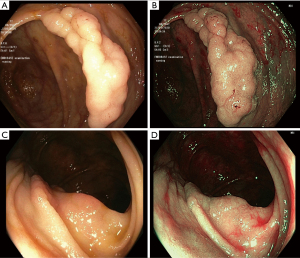
LST-NG has a more regular and homogeneous surface with a risk of submucosal invasion of 7% for tumors less than 20 mm, but this risk increases to 80% for lesions greater than 30 mm (7,8).
Based on the increased risk of submucosal invasion, ESD should be considered for treatment of LST-G-NMs and LST-NGs, especially for larger lesions (>30 mm) (7).
Also the pit pattern (PP) and the vascular pattern (VP) of colorectal lesions can predict submucosal invasion and they can be observed in magnifying endoscopy, with the help of chromoendoscopy, by using indigo carmine solution or methylene blue as contrast, or narrow-band imaging (NBI). NBI is a new technology used to enhance the visualization of the superficial vascular reticulum; the analysis of the distribution of superficial vessels, in the context of neoplastic lesions of the colon-rectum, allows the NBI International Colorectal Endoscopic Classification (NICE), which characterizes hyper-plastic lesions (type 1), adenomas (type 2) and deep invasive submucosal cancers (type 3), by the analysis of color, VP and PP of lesions (9).
Kudo classification classifies the PP of a lesion and it identifies seven patterns, grouped into three categories: (I) non-neoplastic (type I and II), (II) neoplastic low grade (type III), (III) neoplastic high grade (type IV and V).
III and IV types are non-invasive patterns, suggesting a submucosal invasion <1,000 µm, and are candidates for radical endoscopic resection. V type, that can be distinguished in Vi (irregularly arranged surface) and Vn (area of obvious non structure), is highly indicative of submucosal invasion >1,000 µm (5,7), especially the Vn type, as indicated in several studies where the accuracy of Vn-type PP detection for submucosal invasion >1,000 µm is more than 95% (10,11). By contrast, lesions with Vi-type PP include dysplasia and various submucosal carcinomas, therefore it is necessary to analyze it in detail to decide the appropriate therapy (11). Therefore, colorectal neoplasms characterized by type Vi PP according to the Kudo classification and type 2 pattern in NICE classification should be considered for ESD resection, as indicative of superficial invasion of the submucosa, while the identification of disrupted PP and absent or irregularly thickened vessels (Kudo type Vn, NICE type 3) should be predictive of deep submucosal invasion and surgery must be considered (5) (Table 1).
Table 1
| Type | Endoscopic | Ideal treatment |
|---|---|---|
| I | None endoscopic resection | |
| II | None endoscopic resection | |
| IIIS | Endoscopic resection | |
| IIIL | Endoscopic resection | |
| IV | Endoscopic resection | |
| Vi | Endoscopic resection; surgical treatment | |
| Vn | Surgical treatment |
Definitively, ESD can be evaluated for colorectal lesions that cannot be optimally and radically removed by standard techniques of endoscopic resection and for lesions with high suspicion of limited submucosal invasion and low risk of lymph node metastasis, particularly if they are larger than 20 mm (12). ESD is also indicated in case of presence of lesional and perilesional fibrosis caused by previous biopsy sampling, residual tumors after partial endoscopic resections or sporadic tumors associated at chronic inflammatory bowel disease (13).
Colorectal ESD: technique
ESD technique performed in the colorectum is the same used for lesions of the upper gastrointestinal tract (5). ESD is characterized by several steps: thermal marking of the lesion, submucosal injection, circumferential cutting of the mucosa around the lesion, dissection of the submucosa (14). ESD is performed using, generally, a standard or a pediatric colonoscope with water jet function, with a transparent hood attached to the tip of the endoscope for a better exposure of the submucosal layer.
First of all, the lesion should be marked because its margins may become hardly visible after the submucosal injection. The tag is performed using the tip of a knife, through small clot spots around the lesion, about 1–2 mm away from it (15). The second step is characterized by submucosal injection to elevate the lesion from the muscle layer, using a viscous solution, generally a sodium hyaluronate and glycerol solution (5). It’s recommended more viscous injection over the use of normal saline solution, because this one does not provide a long-lasting cushion (14,16). A needle knife or a dual knife is employed to circumferentially incise and delimit the mucosa around the lesion, and dissect it from the connective tissue of the submucosal layer: this is obtained starting from two small incisions in the mucosa on two opposite sides and continuing to incise the mucosa in two directions until reaching the controlateral point and completing the cutting circumference. When the mucosal incision is finished, submucosal dissection is performed by short and controlled cuts, and as the submucosal layer is exposed and separated from the underlying muscle, the submucosal vessels will be identified and they must be adequately coagulated to prevent bleeding (15).
Actually, various techniques are being developed to perform ESD safely and to overcome difficulties coming from particular situations, such as presence of fibrosis in the submucosal layer, angulation of the colon, poor endoscopic maneuverability (17).
The pocket creation method (PCM) was proposed by Hayashi et al. and consists on performing, after an initial mucosal incision, a pocket in the submucosa under the lesion by introducing the endoscope using a transparent hood and needle-type knife. This technique permits a safe dissection because the minimal incision can keep the submucosal layer thick and relatively easy to approach even in situations with poor endoscopic maneuverability (18,19).
The traction method is an alternative technique used to overcome difficulties associated with a poor field of vision of the submucosa. The traction force may be applied using snares and clips, as in clip with line method (20,21), clip with sinker method (22) or clip and snare method (23,24), or grasping forceps (25).
Another alternative to the standard ESD technique, which is spreading especially in the Western countries, consists of a “hybrid” between ESD and EMR (26,27), such as the circumferential submucosal incision EMR (CSI-EMR) and the Hybrid knife-assisted ESD, with the aim of reducing the timing of the procedure and rate of complications, while still obtaining an en bloc resection and reducing the risk of recurrence.
When colonic lesions cannot be removed by endoscopic resection techniques, combined endoscopic and laparoscopic surgery (CELS) can be performed. CELS facilitates the resection of colonic tumors through external manipulation of the bowel wall with laparoscopic instruments, but avoiding intestinal resection; it is applied when the lesions are particularly large or when they are in anatomical area difficult to reach endoscopically (28).
Colorectal ESD: complications
ESD has a higher rate of complications than other endoscopic procedure. Bleeding is the most common adverse event, occurring in a percentage of cases ranging from 5% to 8% and it is more frequent in gastric lesions than in colon-rectum (15). Intra-procedural bleeding is common and depends on minor oozing from submucosal vessels, but it must be treated immediately with the coagulation delivered through hemostatic forceps, coagraspers, argon plasma coagulation or endoclip (29). Bleedings occurring later than 72 h are generally rarer and tend to limit themselves without endoscopic therapies. Perforation is a complication that occurs in a percentage of cases ranging from 3% to 5% and it is more frequent in colorectal lesions (15). Perforation is often recognized intra-procedurally and endoclipping, associated with conservative treatment, such as nasogastric suction, fasting and antibiotics, is usually effective, avoiding surgical treatment (30). For delayed perforation, not identified during an endoscopic procedure, surgery may be necessary. Perforation rate could be decreased with the practice of the technique, using an appropriate knife (31), while it increases in case of colorectal tumors involving diverticula, due to the absence of a muscular layer in them (32). Hong et al. have developed a validated score, called SELF, consisting on four clinical factors (Size, Experience, Location and Fibrosis) to predict the risk of colorectal ESD-induced perforation (33).
Pain after ESD is moderate and persists few days after the procedure, but its frequency is low (29); also the risk of stenosis after ESD is very low (34).
Colorectal ESD: past, present and future
Before the development of ESD technique, EMR was the most used endoscopic technique for early colorectal tumors, and it is still a routine procedure performed all over the world and especially in the Western countries (35). During the years, a lot of works had compared the two techniques and they had highlighted the superiority of the ESD technique both for complete resection and for local recurrence (36-38).
One of the largest case series on colorectal ESD was conducted by Saito et al. on 1,111 colorectal neoplasms in 1,090 patients who underwent ESD from June 1998 to February 2008. This study showed that ESD, if performed by expert operators, can be a valid alternative to surgery and achieves optimal results, significantly superior to all EMR series, with an en bloc resection and curative resection of 88% and 89% respectively.
Perforation occurred during the procedure in 4.9% of patients, and delayed in 0.4% of cases, while postoperative bleeding occurred in 1.5% of cases. The risk of perforation was directly proportional to the size of the lesion and tumor size larger than 50 mm was considered an independent risk factor for complications, while operator experience and a large number of procedures performed at the same institution reduce the adverse events related to the ESD technique (39). Another important advantage of the ESD technique compared to the EMR is that it allows en bloc resection of colorectal lesions larger than 2 cm, preserving a single histological piece for a more accurate anatomical-pathological examination (27). At first, ESD was performed in the upper gastrointestinal tract to remove localized gastric tumors, then it was also applied to resect lesions in the colon-rectum. Fujishiro et al. performed ESD on 200 consecutive colorectal neoplasms and they demonstrated that this technique is applicable in the colon with promising results, with an en bloc resection and R0 resection rates of 91.5% (183/200) and 70.5% (141/200), respectively, even if it’s more appropriate for large flat neoplasms or those with submucosal fibrosis (40).
The R0 resection rate during ESD has progressively improved over the years and ESD has become an increasingly effective technique for the complete resection of large lesions of the colorectum (41).
Lee et al. performed ESD on 1,000 colorectal tumors from October 2006 to August 2011 and showed that ESD allows en bloc resection and R0 resection, with a rate of 97.5% and 91.2% respectively, of large colorectal epithelial tumors and submucosal tumors, and it may gradually replace piecemeal EMR and radical colon resection in the treatment of colorectal neoplasms. However, they described a substantial risk of perforation, corresponding to 5.3% (53/1,000) of cases (42).
Similar results were obtained by Hotta et al., that performed ESD on 146 colorectal lesions in 140 patients from January 2009 to July 2011: the en bloc resection and R0 resection rates were 92.5% and 83.6%, respectively, while perforation and late bleeding occurred in 2.1% and 1.4%, respectively (43).
Several studies have evaluated the safety and efficacy of colorectal ESD and almost all of these are obtained from large series from expert Asiatic centers.
ESD is related to a high percentage of en bloc resection and curative resection rates with an interval between 95.3% to 97.1% and 84.9% to 87.7%, respectively, for gastric localization (44,45) and 88% to 98.3% and 88.6% to 89%, respectively, for the colorectum (39,45).
One of the largest meta analysis, showing that ESD is safe and effective for colorectal tumors was proposed by Akintoye et al. They reviewed 13,833 cases of colorectal cancers in 13,603 patients, treated with ESD from 1998 to 2014. These data were mainly processed in South Korea and Japan and they highlighted about 92% (95% CI, 90-94%) for endoscopic en bloc resection rate and 86% (95% CI, 80–90%) for R0 resection rate in Asian countries, with a risk of immediate and delayed perforation of 4.2% (95% CI, 3.5–5.0%) and 0.22% (95% CI, 0.11–0.46%) respectively, while the risk of significant bleeding was immediate in a 0.75% (95% CI, 0.3–1.8%) and delayed in a 2.1% (95% CI, 1.6–2.6%). By analyzing separately the data from Asian and Western countries, they noticed a difference between them, both in terms of en bloc resection rate and in terms of complications. Particularly, endoscopic en bloc resection rate was 94% (95% CI, 92–95%) for Asia and 82% (95% CI, 76–87%) in Western countries, while immediate and delayed perforation rates were 3.8% (95% CI, 3.1–4.6%) and 0.18% (95% CI, 0.08–0.42%) in Asian countries, and 6.6% (95% CI, 4.6–9.4%) and 1.2% (95% CI, 0.29–4.6%) for Western countries and immediate and delayed major bleeding rates followed the same trend with 0.39% (95% CI, 0.11–1.3%) and 1.8% (95% CI, 1.4–2.4%) for Asia, and 3.3% (95% CI, 1.4–7.6%) and 3.9% (95% CI, 2.5–5.8%) for Western countries (35). Similar results were obtained in a systematic review and meta-analysis of 109 studies by Fuccio et al. in the 97 studies evaluating the standard technique, they yielded a pooled estimate of 91% (95% CI, 89.2–92.5%) for endoscopic en bloc resection rate and 82.9% (95% CI, 80.4–85.1%) for R0 resection rate. However, by using subgroup analysis for the different parts of the world where ESD was performed, they showed that the en bloc resection rate and R0 resection rate were significantly higher in Asian countries versus Western countries, and, particularly, en bloc resection rate was 93% vs. 81.2% respectively and R0 resection rate was 85.6% vs. 71.3% respectively (46).
While there are various case series showing the safety and the efficacy of colorectal ESD, long term outcomes have also started to be published in the last few years (2).
Niimi et al. reviewed 310 cases of colorectal tumors in 290 patients underwent ESD from July 2000 to December 2008. As short terms outcomes, the en bloc resection and the absence of residual disease (R0) were 90.3% and 74.5% respectively, with 14 cases (4.5%) of intra-operative perforation, treated successfully by endoscopic clipping, and 1 case of postoperative perforation needed surgery, while postoperative bleeding occurred in 4 cases (1.3%). The long-term study showed 224 patients in good health with no signs of disease recurrence, concluding that ESD was a valid alternative to colectomy for colorectal lesions that do not present lymph node involvement (47) (Table 2). Also Toyonaga et al., in a longitudinal cohort study in single third level reference center, showed that ESD was associated with high curative resection rates and overall long-term prognosis for patients treated with ESD appeared to be excellent, after performing ESD on 1,635 early gastrointestinal tumors (45). In addition to short and long-term outcomes of colorectal ESD, some studies have also analyzed outcomes in special population, such as the elderly population, showing that elderly patient could potentially have more benefit from a minimally invasive technique for treatment of early gastrointestinal tumors and that outcomes for colorectal ESD in elderly and young patients are equal (48-50). Instead, a lower en bloc resection rate and a higher risk of perforation have been demonstrated in difficult ESD cases, and in particular those associated with severe fibrosis (51,52).
Table 2
| Authors | No. of ESD | En bloc resection, % | Curative resection, % | Intraoperative perforations, % | Delayed perforations, % | Postoperative bleedings, % |
|---|---|---|---|---|---|---|
| Saito et al., 2010, (39) | 1,111 | 88 | 89 | 4.9 | 0.4 | 1.5 |
| Fujishiro et al., 2007, (40) | 200 | 91.5 | 70.5 | 5.5 | 0.5 | 1 |
| Lee et al., 2013, (42) | 1,000 | 97.5 | 91.2 | 5.3 | 0 | 0.5 |
| Hotta et al., 2012, (43) | 146 | 92.5 | 83.6 | 1.4 | 0.7 | 1.4 |
| Akintoye et al.*, 2016, (35) | 13,833 | 92 | 86 | 4.2 | 0.22 | 2.1 |
| Fuccio et al.*, 2017, (46) | 18,764 | 91 | 82.9 | 5.2 | – | 2.7 |
| Niimi et al.*, 2010, (47) | 310 | 90.3 | 74.5 | 4.5 | 0.3 | 1.3 |
*, meta-analysis. ESD, endoscopic submucosal dissection.
Colorectal ESD is now considered the standard therapy of several superficial gastrointestinal lesions in Asian countries, presenting similar efficacy and less invasivity compared with surgery method (2,12). Colorectal ESD could become the reference treatment even in Western countries, but the inexperience of endoscopists and the risk of complications limit its practice (53).
The lack of ESD experience could be associated with increased incidence of adverse events related to the procedure and with a higher risk of incomplete resection (54). ESGE starts the development of a curriculum for ESD to improve the dissemination of this technique in Europe, and the aim of ESD curriculum is to train European endoscopist with the necessary skills in order to be able to practice high quality ESD technique.
ESGE recommends that training in ESD should be considered by experienced and fully trained endoscopists, who already perform EMR and are able to manage complications in endoscopy. ESGE discourages the starting of initial ESD training in humans, and improves practice on ex vivo models and animals, where at least 20 procedures should be performed before human practice. Performance of ESD in human should start after a period of observation and assistance of experts performing the procedure, and it should be done, possibly under the supervision of an ESD-proficient endoscopist, on selected lesions, smaller than 30 mm and localized in the antrum or in the rectum, because they are associated with a lower risk of non curative resection (53).
Colorectal ESD: ESD on ascending colon
ESD technique represents a challenge for all endoscopists, especially for the Western ones, and the difficulty of execution depends on the site to be treated. Particularly, in the lower gastrointestinal tract, the technique is more difficult in the cecum and the right-side of the colon than in the rectum or sigma: this depends on various factors associated with anatomical diversity of the various segments. The colon wall in the right-side is thinner than in the left-side and, especially when air is insufflated, endoscopic resection of lesions is associated with a higher risk of perforation, especially in the cecum, that is dome-shaped and its vertical axis usually approaches the knives, and for this reason, it’s better to use hook knife (55). While the risk of perforation is higher in the cecum and in the sigmoid colon (56), the risk of bleeding is higher in the right-side colon than in the left-side, both for the thinner wall and for the presence of more superficial submucosal arteries (57).
Suzuki et al. identified 325 superficial colorectal tumors in 317 patients dissected with ESD technique in their hospital in a period between January 2009 and June 2013; there were late post-ESD bleedings in 14 lesions from 14 different patients (4.3% of all specimen, 4.4% patients), with an average loss of 2.35 g/dL of hemoglobin. Hemostasis was practiced in all patients with endoscopic clipping. They also showed that, in a univariate analysis, lesions located in the cecum were significantly associated with delayed post-ESD bleeding, while, in a multivariate analysis, the location of a lesion in the cecum was an independent risk factors for late bleeding regardless of other conditions. Therefore, it should be recommended a careful and additional management for patients with lesions located in the cecum, due to the increased risk of early and late bleeding (58).
Few studies have been conducted on the performance of ESD, and of endoscopic resection techniques in general, on the proximal colon.
Arimoto et al. led a prospective multicenter observational trial in the period between March 2015 and April 2016 conducted in 106 patients treated by ESD for colorectal lesion.
Analyzing patient characteristics (age, sex), tumor location, and technical data, such as ESD operation time and frequency of post-colorectal ESD coagulation syndrome (PECS), defined as local abdominal pain corresponding to the site of ESD within 4 days to the procedure and evaluated by a visual analogue scale, they found that female gender, ESD operation time longer than 90 minutes and location of lesion in the cecum were significant risk factors independent of PECS (59).
Also previous studies have reported location in the right-side colon as a possible risk factor for development of PECS (60,61): this can be explained with the more thinness of the colon wall in right side, that results so easily stretchable, therefore heat produced during electrocoagulation may reach more easily the muscularis propria.
Another important aspect to consider is the difficulty that sometimes is found to reach the ascending colon and the cecum, due to individual factors, such as age, female sex, previous abdominal or pelvic surgery, or to features of the colon such as loops, angulations, diverticular disease (62). Therefore, also ESD can be technically difficult in some situations such as paradoxical movement of the ascending colon, and in these cases, the double-balloon endoscope may provide stable endoscopic maneuvering, as showing by Yamashina et al. They reviewed the data of 63 patients with 63 superficial proximal colon tumors treated by balloon-assisted ESD (BAESD) between January 2011 and September 2016 and they demonstrated that balloon-assisted endoscopy achieved safe and effective ESD of colonoscopy difficult proximal colon lesions (63).
When right colon lesions are not removable by endoscopic resection, right colon resection is considered the optimal treatment; a new technique has recently been introduced to avoid intestinal resection using a CELS, that facilitates the resection of colorectal tumors by external manipulation of the bowel wall with laparoscopic instruments (28,64).
Colorectal ESD: conclusion
ESD is a technically challenging procedure, but several studies have shown that it is a safe and effective procedure for the treatment of selected forms of gastrointestinal tumors, even in sites where it is more difficult to perform, such as the ascending colon.
ESD performed on right-side colon is associated with a higher risk of PECS and complications, especially for its anatomical features, although support techniques have been developed to avoid them and facilitate the procedure, such as CELS and BAESD.
ESD is certain to become the method of choice for the local treatment of gastrointestinal lesions in Western countries, once we are able to acquire sufficient experience.
Acknowledgments
Original images of Figure 1 taken from database of AOU Federico II Endoscopi Surgery.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Milone and Ugo Elmore) for the series “Right Colectomy 2.0” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-94). The series “Right Colectomy 2.0” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kitajima K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 2004;39:534-43. [Crossref] [PubMed]
- Ferreira J, Akerman P. Colorectal Endoscopic Submucosal Dissection: Past, Present and Factors Impacting Future Dissemination. Clin Colon Rectal Surg 2015;28:146-51. [Crossref] [PubMed]
- Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015;20:207-39. [Crossref] [PubMed]
- Barel F, Cariou M, Saliou P, et al. Histopathological factors help to predict lymph node metastasis more efficiently than extra-nodal recurrences in sub mucosa invading pT1 colorectal cancer. Sci Rep 2019;9:8342. [Crossref] [PubMed]
- Fuccio L, Ponchon T. Colorectal endoscopic submucosal dissection. Best Pract Res Clin Gastroenterol 2017;31:473-80. [Crossref] [PubMed]
- . The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [Crossref] [PubMed]
- Se Kudo. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc 2008;68:S3-47. [Crossref] [PubMed]
- Uraoka T, Saito Y, Matsuda T, et al. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut 2006;55:1592-7. [Crossref] [PubMed]
- Hayashi N, Tanaka S, Hewett DG, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc 2013;78:625-32. [Crossref] [PubMed]
- Tanaka S, Kaltenbach T, Soetikno R, et al. High-magnification colonoscopy (with videos) Gastrointest Endosc 2006;64:604-13. [Crossref] [PubMed]
- Kanao H, Tanaka S, Chayama K, et al. Clinical significance of type Vi pit pattern subclassification in determining the depth of invasion of colorectal neoplasms. World J Gastroenterol 2008;14:211-7. [Crossref] [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Tanaka S, Kashida H, Saito Y, et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2020;32:219-39. [Crossref] [PubMed]
- ASGE Technology Committee. Endoscopic submucosal dissection. Gastrointest Endosc 2015;81:1311-25. [Crossref] [PubMed]
- Repici A, Pagano N, Arezzo A. Tecnica della Endoscopic Submucosal Dissection. Giorn Ital End Dig 2008;31:59-64.
- Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic submucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017;49:270-97. [Crossref] [PubMed]
- Mizutani H, Ono S, Koike K, et al. Recent Development of Techniques and Devices in Colorectal Endoscopic Submucosal Dissection. Clin Endosc 2017;50:562-8. [Crossref] [PubMed]
- Hayashi Y, Sunada K, Takahashi H, et al. Pocket-creation Method of Endoscopic Submucosal Dissection to Achieve en Bloc Resection of Giant Colorectal Subpedunculated Neoplastic Lesions. Endoscopy 2014;46 Suppl 1 UCTN:E421-E422.
- Hayashi Y, Miura Y, Yamamoto H. Pocket-creation method for the safe, reliable, and efficient endoscopic submucosal dissection of colorectal lateral spreading tumors. Dig Endosc 2015;27:534-5. [Crossref] [PubMed]
- Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc 2012;45:375-8. [Crossref] [PubMed]
- Yamasaki Y, Takeuchi Y, Uedo N, et al. Traction-assisted colonic endoscopic submucosal dissection using clip and line: a feasibility study. Endosc Int Open 2016;4:E51-E55. [PubMed]
- Saito Y, Emura F, Matsuda T, et al. A new sinker-assisted endoscopic submucosal dissection for colorectal cancer. Gastrointest Endosc 2005;62:297-301. [Crossref] [PubMed]
- Yoshida N, Doyama H, Tsuji K, et al. The clip-and-snare method with a prelooping technique during gastric endoscopic submucosal dissection. Endoscopy 2014;46 Suppl 1 UCTN:E611-E612.
- Yamada S, Doyama H, Ota R, et al. Impact of the clip and snare method using the prelooping technique for colorectal endoscopic submucosal dissection. Endoscopy 2016;48:281-5. [PubMed]
- Imaeda H, Hosoe N, Ida Y, et al. Novel technique of endoscopic submucosal dissection by using external forceps for early rectal cancer (with videos) Gastrointest Endosc 2012;75:1253-7. [Crossref] [PubMed]
- Toyonaga T, Man IM, Morita Y, et al. Endoscopic submucosal dissection (ESD) versus simplified/hybrid ESD. Gastrointest Endosc Clin N Am 2014;24:191-9. [Crossref] [PubMed]
- Rizk C, Lankarani A, Wallace M. EMR-ESD del colon retto: il punto di vista degli endoscopisti occidentali. Giorn Ital End Dig 2012;35:121-5.
- Lee MK, Chen F, Esrailian E. Combined Endoscopic and Laparoscopic Surgery May Be an Alternative to Bowel Resection for the Management of Colon Polyps Not Removable by Standard Colonoscopy. Surg Endosc 2013;27:2082-6. [Crossref] [PubMed]
- Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol 2008;14:2962-7. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kobayashi K, et al. Successful nonsurgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal ephitelial neoplasms. Endoscopy 2006;38:1001-6. [Crossref] [PubMed]
- Tanaka S, Oka S, Kaneko I, et al. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc 2007;66:100-7. [Crossref] [PubMed]
- Jimenez-Garcia VA, Yamada M, Ikematsu H, et al. Endoscopic submucosal dissection in management of colorectal tumors near or involving a diverticulum: a retrospective case series. Endosc Int Open 2019;7:E664-E671. [Crossref] [PubMed]
- Hong SN, Byeon JS, Lee BI, et al. Prediction model and risk score for perforation in patients undergoing colorectal endoscopic submucosal dissection. Gastrointest Endosc 2016;84:98-108. [Crossref] [PubMed]
- Hayashi T, Kudo SE, Miyachi H, et al. Management and risk factor of stenosis after endoscopic submucosal dissection for colorectal neoplasms. Gastrointest Endosc 2017;86:358-69. [Crossref] [PubMed]
- Akintoye E, Kumar N, Thompson CC, et al. Colorectal endoscopic submucosal dissection: a systematic review and meta-analysis. Endosc Int Open 2016;4:E1030-E1044. [Crossref] [PubMed]
- Wang J, Zhang XH, Ge J, et al. Endoscopic submucosal dissection vs endoscopic submucosal resection for colorectal tumors: a meta-analysis. World J Gastroenterol 2014;20:8282-7. [Crossref] [PubMed]
- Cai S, Zhong Y, Yao L, et al. Re-evaluation of indications and outcomes of endoscopic excision procedures for colorectal tumors: a review. Gastroenterol Rep (Oxf) 2014;2:27-36. [Crossref] [PubMed]
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectals tumors as determined by curative resection. Surg Endosc 2010;24:343-52. [Crossref] [PubMed]
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multi center study of 1111 colorectal endoscopic submucosal dissections. Gastrointest Endosc 2010;72:1217-25. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kodashima S, et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol 2007;5:678-83. [Crossref] [PubMed]
- Repici A, Hassan C, De Paula Pessoa D, et al. Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy 2012;44:137-50. [Crossref] [PubMed]
- Lee EJ, Lee JB, Lee SH, et al. Endoscopic Submucosal Dissection for Colorectal tumors--1,000 Colorectal ESD Cases: One Specialized Institute's Experiences. Surg Endosc 2013;27:31-9. [Crossref] [PubMed]
- Hotta K, Yamaguchi Y, Ono H, et al. Current opinions for endoscopic submucosal dissection for colorectal tumors from our experiences: indications, technical aspects and complications. Dig Endosc 2012;24:110-6. [Crossref] [PubMed]
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESd Study Group multi center study. Gastrointest Endosc 2009;69:1228-35. [Crossref] [PubMed]
- Toyonaga T, Man-i M, East JE, et al. 1,635 Endoscopic Submucosal Dissection Cases in the Esophagus, Stomach, and Colorectum: Complication Rates and Long-Term Outcomes. Surg Endosc 2013;27:1000-8. [Crossref] [PubMed]
- Fuccio L, Hassan C, Ponchon T, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc 2017;86:74-86.e17. [Crossref] [PubMed]
- Niimi K, Fujishiro M, Kodashima S, et al. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 2010;42:723-9. [Crossref] [PubMed]
- Yoshida N, Naito Y, Sakai K, et al. Outcome of endoscopic submucosal dissection for colorectal tumors in elderly people. Int J Colorectal Dis 2010;25:455-61. [Crossref] [PubMed]
- Tamai N, Saito Y, Tajiri H, et al. Safety and efficacy of colorectal endoscopic submucosal dissection in elders: clinical and follow up outcomes. Int J Colorectal Dis 2012;27:1493-9. [Crossref] [PubMed]
- Uraoka T, Higashi R, Kato J, et al. Colorectal endoscopic submucosal dissection for elderly patients at least 80 years of age. Surg Endosc 2011;25:3000-7. [Crossref] [PubMed]
- Inada Y, Yoshida N, Kugai M, et al. Prediction and treatment of difficult cases in colorectal endoscopic submucosal dissection. Gastroenterol Res Pract 2013;2013:523084 [Crossref] [PubMed]
- Matsumoto A, Tanaka S, Oba S, et al. Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol 2010;45:1329-37. [Crossref] [PubMed]
- Pimentel-Nunes P, Pioche M, Albéniz E, et al. Curriculum for endoscopic submucosal dissection training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2019;51:980-92. [Crossref] [PubMed]
- Coman RM, Gotoda T, Draganov PV. Training in endoscopic submucosal dissection. World J Gastrointest Endosc 2013;5:369-78. [Crossref] [PubMed]
- Lee EJ, Lee JB, Choi YS, et al. Clinical risk factors for perforation during endoscopic submucosal dissection (ESD) for large-sized, nonpedunculated colorectal tumors. Surg Endosc 2012;26:1587-94. [Crossref] [PubMed]
- Kim ER, Chang DK. Management of Complications of Colorectal Submucosal Dissection. Clin Endosc 2019;52:114-9. [Crossref] [PubMed]
- Choo WK, Subhani J. Complication rates of colonic polypectomy in relation to polyp characteristics and techniques: a district hospital experience. J Interv Gastroenterol 2012;2:8-11. [Crossref] [PubMed]
- Suzuki S, Chino A, Kishihara T, et al. Risk factors for bleeding after endoscopic submucosal dissection of colorectal neoplasms. World J Gastroenterol 2014;20:1839-45. [Crossref] [PubMed]
- Arimoto J, Higurashi T, Nakajima A, et al. Risk factors for post-colorectal endoscopic submucosal dissection coagulation syndrome: a multi center, prospective, observational study. Endosc Int Open 2018;6:E342-E349. [Crossref] [PubMed]
- Jung D, Youn YH, Jahng J, et al. Risk of electrocoagulation syndrome after endoscopic submucosal dissec tion in the colon and rectum. Endoscopy 2013;45:714-7. [Crossref] [PubMed]
- Yamashina T, Takeuchi Y, Uedo N, et al. Features of electrocoagulation syndrome after endoscopic submucosal dissection for colorectal neoplasm. J Gastroenterol Hepatol 2016;31:615-20. [Crossref] [PubMed]
- Benvenuti S, Iori A, Chilovi F, et al. La colonscopia difficile. Giorn Ital End Dig 2011;34:207-10.
- Yamashina T, Hayashi Y, Sakamoto H, et al. Balloon-assisted endoscopy facilitates endoscopic submucosal dissection of difficult superficial proximal colon tumors. Endoscopy 2018;50:800-8. [Crossref] [PubMed]
- Yan J, Trencheva K, Lee SW, et al. Treatment for right colon polyps not removable using standard colonoscopy: combined laparoscopic-colonoscopic approach. Dis Colon Rectum 2011;54:753-8. [Crossref] [PubMed]
Cite this article as: Maione F, Chini A, Aprea G, Dinuzzi VP, De Palma GD. Indication and results of endoscopic submucosal dissection for right located lateral spreading tumors. Ann Laparosc Endosc Surg 2021;6:46.


