Transabdominal preperitoneal (TAPP) inguinal hernia repair: how we do it
Introduction
Inguinal hernia repair is one of the most common procedures performed around the world; around 20 million herniorrhaphies are done each year (1). However, laparoscopic hernia repair was introduced over 27 years ago; most hernias are still repaired with an open anterior approach (2,3).
Although laparoscopic techniques have shown some advantages over open approaches, such as lower incidence of wound infection and less pain (4), they also have disadvantages, such as a steeper learning curve and a higher complication rate early in a surgeon’s experience (5).
The description of the critical view of the myopectineal orifice standardized the dissection and posterior repair of the inguinal hernia using laparoscopic surgery (6). Although essential technical principles that contribute to a successful laparoscopic hernia repair are conserved among hernia repair experts, precise details vary per surgeon’s preference.
The surgical details and technique related to these principles and tips & tricks will be described in the following work.
Patient selection and workup
Patients are evaluated paying attention to risk factors related to abdominal wall hernia such as smoking, obesity, lung pathologies, and urinary tract diseases, and factors related to potential hernia complications such as pain, intestinal obstruction symptoms, or skin changes (7).
There is evidence that suggests that a 4-week period of prehabilitation is useful in most patients. We follow the STRONG Guidelines, established by the American College of Surgeons, to optimize the patient’s health before surgery (8).We are reluctant to operate patients that smoke or have a BMI >35 in the elective setting.
A comprehensive evaluation and physical examination of the abdominal wall are performed to confirm the diagnosis and detect any other associated abdominal wall hernia. Carrying out an abdominal wall ultrasound (US) is sufficient in most cases; we only request contrast-enhanced-Valsalva CT scan on selected cases.
Preoperative preparation
Urinary catheter
Always empty the bladder; only in selected cases (e.g., significant hernia defects, recurrent hernia), a Foley’s catheter is left throughout the surgery.
Skin preparation and preoperative antibiotics
According to local guidelines, we use traditional antiseptic cleansing agents either povidone iodine or, Chlorhexidine-alcohol (CHA) in the surgical field, and one preoperative dose of antibiotic is used as our local hospital guidelines mandate.
Patient position and surgical team position
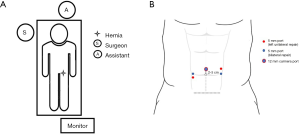
Patient is placed in a supine position, both arms tucked close to the body and fixed to the operating table. Trendelenburg with side inclination, contralateral to the hernia defect, and mild flex are given when the surgery starts. The surgical team is organized as in Figure 1A.
Equipment
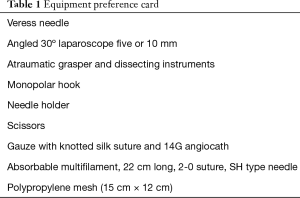
Adequate selection of the instrumentation is vital, as listed on our equipment preference card in Table 1.
Full table
Surgical procedure (Video 1)
Access and port position
The first incision is done 2–5 cm above the umbilical scar. According to the best indication that fits the patient, access to the peritoneal cavity, and pneumoperitoneum is done with the use of a Veress needle or open technique. A 12-mm trocar is placed, and a 30-degree laparoscope is inserted. Under direct vision, two additional 5 mm trocars are placed in each flank, in a horizontal plane with the umbilicus. When doing a unilateral hernia repair, the ipsilateral port is placed 1 cm above the umbilicus level, and the contralateral port 1 cm under the umbilicus level (Figure 1B).
Transversus abdominis plane (TAP) block
Under the direct laparoscopic vision, a TAP block is placed on the affected side. Using as an anatomical landmark, the anterior superior iliac spine (ASIS), 2 cm cephalad the aponeurotic transverse arch is identified, as well as the muscle fibers of the transverse muscle, then 15 mL of ropivacaine + 10 mL of lidocaine are applied in the pre-transversalis space.
Before starting the inguinal dissection
Once in position, the small bowel should be moved away from the surgical area; if the hernia defects have content, they should be reduced by direct careful grasping or external abdominal wall manual pressure. If there is evidence of adhesions to the peritoneal flap (more frequent in the left side), we favor the adhesiolysis with cold scissors to remove any weight to the peritoneal flap.
Peritoneal flap creation
The aim is to start 5 cm above the upper limit of the hernia defect, and this is very relevant since we want to put a mesh wider than 12 cm. First, the peritoneum is incised 2 cm above the ASIS, the peritoneum is pulled away (down and medial) with the non-active hand and CO2 is allowed to enter the pre-transversalis space, allowing pneumodissection and plane separation. The flap cut continues lateral to medial in a horizontal manner, and upon reaching the medial umbilical ligament, a change of direction is made towards a cephalad direction and parallel to the medial umbilical ligament, resembling a hockey stick. The right hernia is easier for the right-handed surgeon since the active hand must always be the most lateral one.
Completing the critical view of the myopectineal orifice
Dissection is performed in the preperitoneal avascular plane between the peritoneum and the transversalis fascia. At the first 2 cm of dissection, the inferior epigastric vessels must be identified as landmarks to continue a safe dissection. The area of dissection is divided into three zones, according to posterior anatomy of the inguinal region and a didactic definition called “Inverted Y,” the “three zones” (Figure 2), and “Five Triangles” described by Furtado et al. (9) and aiming to complete, in all the cases, the nine steps described by Daes and Felix (6).

Zone 2 is located medial to the inferior epigastric vessels and corresponds to the direct hernias’ location. In our opinion, this is the “easy part”. Dissection should extend to the Retzius space until the identification of ipsilateral Cooper’s ligament (CL) and pubic symphysis. While dissecting this zone, one should stay medial to epigastric vessels, take care not to damage the bladder, located in the inferior and lateral border of the screen view, and use the anterior rectal muscle an anatomical reference, seen in the upper edge of the screen. Usually, this space is created with blunt dissection because of the loose areolar tissue; both instrument tips may be used with small circular arm movements, resembling swimming or clapping. The dissection medial to the epigastric artery should be in the parietal compartment of the pretransversal space protecting the bladder of the contact with the mesh. Dissection must be extended to the contralateral CL and 1–2 cm below the pubis and CL and using the obturator fat pad-canal as a landmark to have sufficient space for an adequate sized mesh to fit. A direct hernia should be found during the dissection of this zone. The hernia sac should be mobilized by traction and counter-retraction; once the transversalis fascia is identified and grabbed, it must be separated from the hernia sac in a “pulling a rope” manner. When big direct hernias are found, we suggest the fixation of the pseudo sac to the CL or rectal muscle to potentially decrease the seroma formation. We do not favor the primary closure of the direct defect because there is a chance of taking the cord elements within the suture line.
Zone 1 is located lateral to the inferior epigastric and spermatic vessels. While “parietalization” of the preperitoneal fat covers the nerves located in the pain triangle, muscle fibers must not be exposed, and dissection must stay before de preperitoneal fat to avoid nerve damage and blood oozing. The lateral border of this dissection is the ASIS, and the posterior limit is the psoas muscle. This step is mandatory because the inferolateral corner of the mesh should be correctly positioned in this pocket to avoid indirect recurrence or inferior mesh folding.
Zone 3 corresponds to the step that requires the most attention because it is the mobilization of the peritoneum over the vas deferens, spermatic vessels, and external iliac vessels. To complete zone 3 more efficiently, zone 1 and 2 should be dissected entirely previously. The peritoneum of these structures is removed by tractioning the peritoneal flap and counter-traction of the elements of the spermatic cord. This movement can be called the “parietalization of the elements.” The vas deferens should be moved with a “no-touch or grasp” policy. In women, the round ligament of the uterus should be cut further away from the internal orifice to avoid injuring the genitofemoral nerve at this location. This dissection should be in the visceral compartment of the pre-transversalis space, protecting the nerves of the contact with the mesh. It is essential to visualize the entrance of the round ligament or vas deferens and spermatic cord elements to the deep inguinal ring, before attempting to reduce the hernia sac. Always verify the absence of cord lipoma inside the inguinal canal. Dissection between CL and the iliac vein must be done to rule out a femoral hernia.
Mesh application and fixation
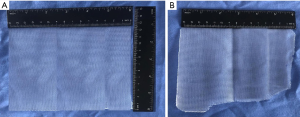
We use polypropylene medium/heavyweight and 15 cm × 12 cm size mesh in most patients (Figure 3). For big direct hernias, we favor a 17 cm × 12 cm mesh. The mesh is rolled up and inserted through the umbilical port. For fixation, either by tackers or suture, the safe zones (CL, ipsilateral rectal muscle) must be taken into account. Nowadays, we tend to only fixate the mesh on selected cases (M3).
Peritoneal flap closure
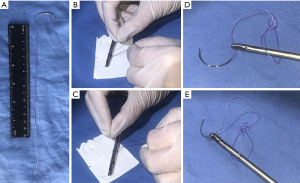
We do intracorporeal suture, with adequate cost-benefit and low pain risk. Decrease the pressure to 8 mmHg and start from lateral to medial, use a 2-0 multifilament, 26 mm, 1/2 needle, 22 cm long suture with a loop at the end (Figure 4), and a mattress-type surgeon closure is performed. In approximately ten passes of suture (3–5 min), the flap must be airtight closed.
Drains and catheters
The bladder catheter must be removed at the end of the procedure if it had been left, and intra-abdominal drains are not routinely used.
Fascia and skin closure
The 12 mm trocar fascia is closed; absorbable monofilament suture and a CT2 needle are preferred. The skin is closed with subdermal stitches of an absorbable suture.
Postoperative management
The average surgical time for a unilateral hernia is 45 min with an in-hospital stay of 24 hours. A liquid diet is indicated 4 hours after surgery, acetaminophen 1 gr quad, ketorolac 10 mg tid for pain control, and preventive emesis prophylaxis are given.
Elastic compression stockings are placed, and low molecular weight heparin is added if necessary. Incentive spirometer and early ambulation are instructed. We recommend using an ice pack four times a day, the first 48 hours, and maintaining the use of a hernia support tensor or tight underpants for 4–6 weeks.
As outpatients, we prescribe 1 gr of acetaminophen every 6 hours for 7 days and 15 mg of meloxicam once a day for 3 days, and 75 mg of pregabalin for 2 weeks, for pain control. The patient is scheduled with two postoperative consults on days 10 and 21. Patients can safely ambulate on the evening of the surgery, with no medical or physical restrictions on activity after 7 days. Returning to regular exercise as soon as possible will most likely enhance the recovery.
Tips and tricks
- Gauze with knotted long silk suture is introduced through the camera 12 mm port and allows it to be pulled out without the need of another 12 mm port.
- Peritoneal flap closure with looped end suture: make a loop at the end of a conventional multifilament suture to avoid the first knot.
- CO2 evacuation in the inguinal area: before closing the flap airtight, insert a 14G angiocath to evacuate CO2 and visualize an adequate mesh position.
- Flattening the learning curve: deep knowledge of anatomy, sticking to the principles, steps, and rules is mandatory. Continuous training, mentoring, online courses, video review, cadaver workshops, and the monitoring of Facebook groups such as International Hernia Collaboration will help the surgeon achieve the best results.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at http://dx.doi.org/10.21037/ales-20-109
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-109). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient. The local ethics committee ruled that no formal ethics approval was required in this case.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burcharth J, Pedersen M, Bisgaard T, et al. Nationwide prevalence of groin hernia repair. PLoS One 2013;8:e54367. [Crossref] [PubMed]
- Köckerling F, Simons MP. Current Concepts of Inguinal Hernia Repair. Visc Med 2018;34:145-50. [Crossref] [PubMed]
- Claus C, Furtado M, Malcher F, et al. Ten golden rules for a safe MIS inguinal hernia repair using a new anatomical concept as a guide. Surg Endosc 2020;34:1458-64. [Crossref] [PubMed]
- Bittner R, Schwarz J. Inguinal hernia repair: current surgical techniques. Langenbecks Arch Surg 2012;397:271-82. [Crossref] [PubMed]
- Novitsky YW, Czerniach DR, Kercher KW, et al. Advantages of laparoscopic transabdominal preperitoneal herniorrhaphy in the evaluation and management of inguinal hernias. Am J Surg 2007;193:466-70. [Crossref] [PubMed]
- Daes J, Felix E. Critical View of the Myopectineal Orifice. Ann Surg 2017;266:e1-e2. [Crossref] [PubMed]
- Petro CC, Prabhu AS. Preoperative Planning and Patient Optimization. Surg Clin North Am 2018;98:483-97. [Crossref] [PubMed]
- Strong for Surgery. Optimizing health prior to surgery. Available online: https://www.facs.org/quality-programs/strong-for-surgery
- Furtado M, Claus CMP, Cavazzola LT, et al. Systemization of laparoscopic inguinal hernia repair (TAPP) based on a new anatomical concept: inverted y and five triangles. Arq Bras Cir Dig 2019;32:e1426. [Crossref] [PubMed]
Cite this article as: Farell Rivas J, Ruiz-Funes Molina AP, Meza Carmona J. Transabdominal preperitoneal (TAPP) inguinal hernia repair: how we do it. Ann Laparosc Endosc Surg 2021;6:12.