Chyle leak post laparoscopic cholecystectomy: a case report, literature review and management options
Introduction
Gallstone disease is a highly prevalent problem worldwide. Laparoscopic cholecystectomy (LC) is currently a well-accepted operation to deal with symptomatic gallstones, with more than 300,000 such operations being performed in USA each year (1). Its postoperative complications have been well described in the literature, including bile leak and bile duct injury, although this procedure is usually associated with low morbidity and mortality.
Chylous ascites is a rare complication of many abdominal and thoracic operations, especially where retroperitoneal and mediastinal dissection has been undertaken. It carries significant morbidity due to the loss of lymph fluid rich in triglycerides, lymphocytes, immunoglobulins and electrolytes. A prompt diagnosis and initiation of treatment is necessary. Our current understanding and management of abdominal chyle leak is derived from cases that occur in the setting of oncological resections in the retroperitoneum. It is most unusual in the setting of an uncomplicated LC.
We report the sixth case of chyle leak after a LC performed for acute cholecystitis. In an attempt to understand this phenomenon we undertake a literature review, propose pathogenesis, diagnostic workup and management options for this rare complication. We suggest a step-up approach to the management of such leaks.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/ales-20-99).
Case presentation
A 48-year-old male presented acutely with a day history of right upper quadrant pain associated with nausea. He has a history of ankylosing spondylitis managed with etanercept injections every 2 weeks.
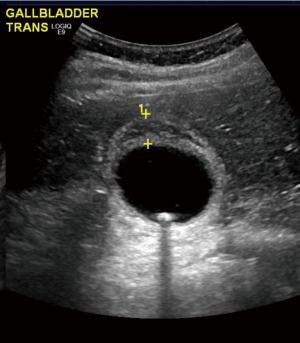
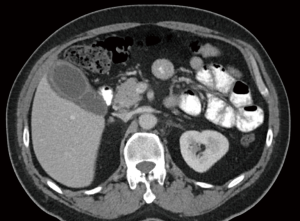
On examination, his vitals were within normal limits. His abdomen was tender in the right upper quadrant with no palpable masses. Murphy’s sign was positive. Laboratory investigations showed a normal white cell count (WCC), C reactive protein (CRP), liver function tests and lipase levels. A computed tomography (CT) scan showed a distended gallbladder with pericholecystic fluid (Figure 1). An ultrasound again revealed a thickened and oedematous gallbladder wall up to 1.2 cm (Figure 2), with two mobile gallstones and no bile duct dilatation. He was diagnosed with acute cholecystitis and commenced on intravenous antibiotics.
The patient underwent a LC later that day. Intraoperatively he had an acutely inflamed gallbladder. The hepatocystic triangle was dissected, with clear exposure of the cystic duct. The cystic artery was exposed after the cystic node was reflected cranially and its attachments taken down with monopolar diathermy. The cystic artery was clipped before being cut. An intraoperative cholangiogram was performed showing no abnormal findings before the cystic duct was doubly clipped. The gallbladder was dissected off the cystic plate and exteriorised via a retrieval bag. A closed-suction silicone drain was left in the subhepatic space as per the surgeon’s practice in the setting of acute cholecystitis. Postoperative pathology would later confirm acute cholecystitis with cholelithiasis.
He was commenced on an unrestricted diet immediately after the surgery and a slightly milky-white drain effluent was noted on the first postoperative day. The patient was clinically asymptomatic with no pain, fever or signs of peritonitis. The drain fluid was sent off for analysis which showed a raised triglyceride concentration (23.2 mmol/L) and normal bilirubin and amylase, with a serum triglyceride of 1.5 mmol/L, i.e., drain/serum TG ratio 15.5. Microbiological analysis showed polymorphs in the drain fluid with no organism being cultured. Hence, he was diagnosed with a chyle leak, rather than bile leak or infected collection. His WCC and liver function tests remained normal throughout the postoperative period. The CRP levels peaked at 42 mg/L on the first postoperative day and gradually improved.
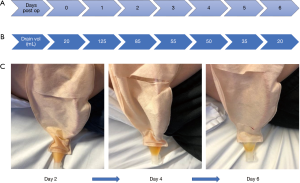
The drain volume was 125 mL on the first day (Figure 3A). He was started on a fat-free diet which he tolerated. The subsequent drainage volume improved (Figure 3B). The content of the drain fluid gradually became serous on postoperative day 4 (Figure 3C). Resolution of the chyle leak was confirmed by repeated testing of the drain fluid which demonstrated normal triglyceride concentration (1.3 mmol/L) (Table 1), with drain/serum triglyceride ratio of 0.9. The drain was removed and he was discharged home on day 6 on a low-fat diet for another week. He had no further complications during his hospital stay or after discharge.

Full table
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
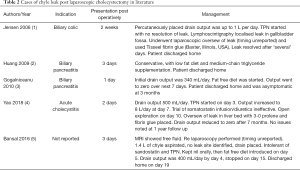
Whilst complications related to bile duct and hepatic artery injuries after LC are uncommon, they have been well documented in the literature. In comparison, we have identified only 5 reported cases of chyle leak after LC for calculous disease making it an exceedingly rare complication (Table 2).
Full table
Chyle leaks are usually of low volume and are self-limiting. However high-volume leaks can cause significant morbidity. Those patients are prone to infections and sepsis as the lymph fluid is rich in lymphocytes. Deficiencies in protein, vitamins, and calories can occur, along with electrolyte imbalances (6). In some cases mortality rates up to 70% have been associated with this complication (7).
The mechanism of a chyle leak following a cholecystectomy is possibly explained by the lymph drainage pattern of the gallbladder. Lymph from the latter drains into the first-order nodes, either the cystic or the pericholedochal node(s) (8). From here there are 3 pathways described: (I) right (more predominant) route which descends along the common bile duct to join the posterosuperior pancreaticoduodenal node(s); (II) left route which runs medial to the hepatoduodenal ligament following the common hepatic artery before reaching the nodes around the coeliac trunk; (III) mesenteric route which flows along the portal vein to the nodes around the superior mesenteric artery (9,10).
However, during an uncomplicated LC, damage to minor lymphatics annexed to the cystic node can potentially happen and would explain minor postoperative chyle leaks, most of which are likely to remain subclinical. A cholecystectomy done for an impacted stone causing chronic cholecystitis whereby the dissection is undertaken more centrally, i.e., in close proximity to the bile duct or an anomalous hepatic artery may lead to inadvertent injury to the lymph outflow along the right or left routes mentioned above. Alternatively it has been suggested that an anatomical lymphatic variant may exist which can predispose some patients to a chyle leak (11). Others have suggested transient mechanical compression of lymphatics by an inflamed pancreas (12). Interestingly, as with our patient, none of the previously reported cases were technically difficult (Table 2).
Diagnosis of a chyle leak can be difficult. Often patients report non-specific symptoms, including abdominal distension, discomfort, or nausea. It may also present as a milky discharge from the wound or the drain (13). Analysis of the fluid samples from the abdomen will help confirm the diagnosis. The presence of a milky fluid in the peritoneal cavity with a triglyceride count greater than 110 mg/dL (1.2 mmol/L) is characteristic of chylous ascites (1). The fluid is typically odourless, alkaline, sterile, rich in lymphocytes but poor in bilirubin and amylase. Furthermore, drain fluid/serum ratio for triglycerides is usually greater than 1.0. In our case, the characteristic appearance of the post-operative drain fluid, with its triglyceride content and drain/serum triglyceride ratio allowed us to make the diagnosis. Alternatively, the diagnosis could be confirmed with chylomicron testing, which unfortunately cannot be processed by our laboratory.
Different imaging modalities can aid in the diagnosis. On CT, it will appear as a low attenuated fluid with a possible fat-fluid level (13). Lymphangiography is traditionally the method employed to locate leaks, but has fallen out of favour due to its invasive nature, technical difficulty and adverse effects associated with the oil-based contrast used. Some studies have shown its usefulness in the treatment of refractory leaks due to the inflammatory reaction of Lipiodol when extravasated (14). Lymphoscintigraphy is able to provide the initial diagnosis of a chyle leak but has had varying success in the exact localisation (7). It involves injecting technetium (99Tc) labelled colloid into the dermis of the interdigital web spaces which gets taken up into the lymphatic channels, and therefore may be visualised as collecting at the site of the leak. It has the advantage of being less invasive with less associated adverse reactions. Some studies advocate for its use over lymphangiography, particularly in selecting patients for surgical repair (2,13). Imaging should be reserved for cases where the diagnosis is unclear or as a localisation tool whilst intervention is being planned. Interestingly, in our literature review (Table 2) among 2 cases that did not settle with conservative means only the one who underwent lymphoscintigraphy before surgical intervention had a more favourable postoperative outcome (1).
In our case we did not perform any postoperative imaging as the leak settled and there were no signs of undrained infected collection. Otherwise we would have organised a CT before contemplating percutaneous drainage in the first instance before moving to lymphoscintigraphy in case of persistent leakage.
The management of chyle leaks after LC draws upon our experiences with chylous ascites and chylothoraces. However, there is no strong consensus with regards to treatment due to a lack of evidence from controlled trials. It is agreed that therapy should be initiated swiftly in cases of high-volume leaks. Management can be broadly divided into 2 categories: conservative and surgical. Conservative treatment should be started first, with surgical intervention reserved for more severe or persistent cases.
Paracentesis is useful in symptomatic chylous ascites with no drain in situ. Repeated paracentesis should be avoided due to the risk of infection, increased nutritional losses and possibility of prolonging the leak (13,15).
Conservative management revolves around reducing enteric lymph flow and replacing any fluid, protein or electrolyte losses (13). Lymph normally flows through the thoracic duct at an average 1 mL/kg/h but increases up to 200 mL/kg/h after ingestion of a fatty meal (2). The use of short term total parenteral nutrition (TPN) is the most reliable and immediate way to decrease intestinal lymph flow allowing the damaged lymph channels to heal. Studies have shown TPN alone can resolve cases in 77% to 100% of cases (16). It is advantageous to maintain the benefits of enteral feeding by placing the patient on a low fat, high protein-based diet with medium-chain triglycerides (MCT) supplementation. MCTs bind to albumin and enter the portal system directly bypassing the lymphatic system, whereas long-chain triglycerides are absorbed directly into the intestinal lymphatics (17). However, the literature has reported lower success rates compared to TPN (16). There are no randomised trials to compare these. In the acute inpatient setting or in leaks with significant volumes, it may be suitable to place the patient on TPN for rapid resolution, while reserving MCT diets for low volume leaks less than 100 mL/day (11,18). On discharge, the continuation of a low-fat diet with medium-chain triglycerides for a few months has been suggested to prevent recurrence.
In our case we started a normal diet as per our practice after an uneventful cholecystectomy. This certainly compounded the chyle leak. However, a prompt diagnosis of the complication and institution of a fat free diet helped with rapid resolution of the low volume leak. The risk of a chyle leak after LC for calculous disease is very low, as evidenced by 5 cases in the literature search in Table 2. There might be a role for an upfront restrictive diet in high risk patients for example those having a complex biliary dissection and especially if there is intraoperative chyle leakage noted. Those patients should be nil by mouth with slow progression to fat free/high protein/MCT diet while observing for chyle leakage.
Pharmacological agents may be added to the treatment regimen if dietary modification alone is not effective in reducing the chylous ascites. The reported usefulness is variable. Pancreatic lipase inhibitors (e.g., orlistat) and synthetic somatostatin analogues (e.g., octreotide) may help decrease triglyceride absorption and enteric chyle flow (19). Some studies have shown a significant improvement in output after a few days of subcutaneous administration or continuous infusion, whereas others have had less success (13). The addition of octreotide to TPN or a medium-chain triglyceride diet has been shown to resolve leaks significantly earlier in hepatopancreaticobiliary surgeries and nephrectomies (16,18). Somatostatin therapy can be started at 100 µg 3 time a day. Additionally, antibiotic prophylaxis is also advocated to prevent superimposed infection (1).
Surgical intervention is another cornerstone of management, especially since conservative approaches are not always effective. As expected, there is a large paucity of evidence in the timing of reoperation for chyle leaks post LC. Important clinical factors to consider in each case would be the current clinical status of the patient, the severity of the chyle leak (especially if >500 mL per day) and the original surgical procedure. Also chyle leaks that are clearly seen on lymphoscintigraphy may be unlikely to resolve with non-operative management (2). The aims of surgical intervention are drainage of the leaked chyle followed by ligation of the culprit lymphatic. A laparoscopic approach can be safe and feasible in experienced hands, with resolution achieved if the leak is clearly visualised at the time of reoperation. Fibrin glue can also be used as an adjunct (1,13). Nonetheless, the timing of surgical intervention is not defined, especially since some leaks can take several weeks to resolve on conservative treatment. Currently, the decision to return to theatre is strongly based on surgeon discretion.
Various other treatments for chylous ascites has been reported in the literature. However, these are not commonly used either due to adverse effects or lack of evidence for success. Etilefrine, a sympathomimetic used in postural hypotension, has been shown to reduce chyle leaks by acting on smooth muscles to constrict the lymphatics (20). Peritoneovenous shunts can reduce the nutritional, fluid and immunological losses from lymph leaks, but predispose the patient to disseminated intravascular coagulopathy, sepsis, and fat emboli (13). As such, shunts have fallen largely out of favour.
Our review suggests that the volume of leak may predict likelihood of resolution with conservative treatment alone (Table 2). Of the 5 cases, 3 (2,3,5) settled simply with low fat diet, without TPN or ongoing octreotide use. Interestingly they all had less than 1 L of leak per day after initial drainage. The other cases (1,4) had >1 L/day output and did not settle with TPN or somatostatin infusion and eventually required surgical intervention. Resolution of our low volume leak without the need for TPN or intervention in line with the review.
This report only describes the case of a low volume chyle leak that settled with conservative means. However, we highlight it in the context of a literature review with a detailed summary of similar cases. We include a pathogenesis of this rare complication and provide an up to date evidence-based approach to the investigative and management options available.
This case shows that chyle leaks, although very rare, can occur even in technically uncomplicated LC. Our recommended management protocol would be to commence the patient on a low fat, high protein, medium chain triglyceride diet and assess its efficacy as we did for our case. If the output is >500 mL per day despite those measures, then we suggest the commencement of TPN with the inclusion of somatostatin therapy, escalating its dose as needed. Imaging should be done early in these cases to localise of the leak. Surgical intervention and ligation of the leaking lymphatic should then be attempted early before inflammation sets in and makes reoperation hazardous.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/ales-20-99
Peer Review File: Available at http://dx.doi.org/10.21037/ales-20-99
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-99). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jensen EH, Weiss CA 3rd. Management of chylous ascites after laparoscopic cholecystectomy using minimally invasive techniques: a case report and literature review. Am Surg 2006;72:60-3. [Crossref] [PubMed]
- Huang YM, Chen JH, Liu SH, Lin MT. Chyle leakage after laparoscopic cholecystectomy for acute biliary pancreatitis: a case report. Hepatogastroenterology 2009;56:39-42. [PubMed]
- Gogalniceanu P, Purkayastha S, Spalding D, et al. Chyle leak following laparoscopic cholecystectomy: a rare complication. Ann R Coll Surg Engl 2010;92:W12-4. [Crossref] [PubMed]
- Yao BZ, Li L, Jiang M, et al. Refractory chyle leakage after laparoscopic cholecystectomy for gallstone disease: A case report and literature review. Medicine (Baltimore) 2018;97:e9604 [Crossref] [PubMed]
- Bansal A, Bansal AK, Bansal V, et al. Spontaneous chylous ascites after laparoscopic cholecystectomy: a case report. Int Surg J 2016;3:408-10. [Crossref]
- Cárdenas A, Chopra S. Chylous ascites. Am J Gastroenterol 2002;97:1896-900. [Crossref] [PubMed]
- Aalami OO, Allen DB, Organ CH Jr. Chylous ascites: a collective review. Surgery 2000;128:761-78. [Crossref] [PubMed]
- Shirai Y, Wakai T, Hatakeyama K. Radical lymph node dissection for gallbladder cancer: indications and limitations. Surg Oncol Clin N Am 2007;16:221-32. [Crossref] [PubMed]
- Sato T, Ito M, Sakamoto H. Sakamoto, Pictorial dissection review of the lymphatic pathways from the gallbladder to the abdominal para-aortic lymph nodes and their relationships to the surrounding structures. Surg Radiol Anat 2013;35:615-21. [Crossref] [PubMed]
- Uesaka K, Yasui K, Morimoto T, et al. Visualization of routes of lymphatic drainage of the gallbladder with a carbon particle suspension. J Am Coll Surg 1996;183:345-50. [PubMed]
- Cheung CX, Kelly ME, El Tayeb O, et al. Chylous ascites post open cholecystectomy after severe pancreatitis. JOP 2012;13:278-81. [PubMed]
- Ben-Ami H, Nagachandran P, Assalia A, et al. Acute transient chylous ascites associated with acute biliary pancreatitis. Am J Med Sci 1999;318:122-3. [Crossref] [PubMed]
- Leibovitch I, Mor Y, Golomb J, et al. The diagnosis and management of postoperative chylous ascites. J Urol 2002;167:449-57. [Crossref] [PubMed]
- Kawasaki R, Sugimoto K, Fujii M, et al. Therapeutic effectiveness of diagnostic lymphangiography for refractory postoperative chylothorax and chylous ascites: correlation with radiologic findings and preceding medical treatment. AJR Am J Roentgenol 2013;201:659-66. [Crossref] [PubMed]
- Olthof E, Blankensteijn JD, Akkersdijk GJ. Chyloperitoneum following abdominal aortic surgery. Vascular 2008;16:258-62. [Crossref] [PubMed]
- Weniger M, D'Haese JG, Angele MK, et al. Treatment options for chylous ascites after major abdominal surgery: a systematic review. Am J Surg 2016;211:206-13. [Crossref] [PubMed]
- Garrett HE Jr, Richardson JW, Howard HS, et al. Retroperitoneal lymphocele after abdominal aortic surgery. J Vasc Surg 1989;10:245-53. [Crossref] [PubMed]
- Kuboki S, Shimizu H, Yoshidome H, et al. Chylous ascites after hepatopancreatobiliary surgery. Br J Surg 2013;100:522-7. [Crossref] [PubMed]
- Kalomenidis I. Octreotide and chylothorax. Curr Opin Pulm Med 2006;12:264-7. [Crossref] [PubMed]
- Guillem P, Papachristos I, Peillon C, et al. Etilefrine use in the management of post-operative chyle leaks in thoracic surgery. Interact Cardiovasc Thorac Surg 2004;3:156-60. [Crossref] [PubMed]
Cite this article as: Ong F, Das A, Rajkomar K. Chyle leak post laparoscopic cholecystectomy: a case report, literature review and management options. Ann Laparosc Endosc Surg 2021;6:25.