Is air leak testing really necessary after stapled anterior resection?—a case series and a literature review
Introduction
Air leak testing (ALT) has been routinely performed following hand-sewn anastomosis after anterior resection (AR) and for obvious reasons as it might take extra suturing for the surgeon to obtain a satisfactory seal. Expectedly and for no other reason than continuing a well-established practice, ALT is currently still used with stapled anastomosis. A Dephi meeting of colorectal surgeons of Great Britain and Ireland found that all their surveyed members agreed that an ALT ought to be performed (1). The rationale is that it tests the airtightness of a newly fashioned anastomosis and hence theoretically helps reduce postoperative leaks. Whilst there is ample evidence to suggest that ALT was helpful in leak reduction after hand-sewn anastomosis, the real impact on the postoperative leak rate post stapled anastomosis in our current era is hard to fully assess. The body of evidence includes low volume level 4 studies with numerous confounders that are likely to influence the leak rate. In fact, there are numerous complex factors that affect leaks after ARs for rectosigmoid pathologies, which are difficult to standardize in order to assess the true usefulness of the ALT. There has been some suspicion raised regarding the morbidity of increased intraluminal pressure on a newly fashioned anastomosis while performing an ALT.
Is it safe to construct a stapled anastomosis after an AR without an ALT? Can a carefully fashioned stapled anastomosis obviate the need for intraoperative ALT if rigorous attention is paid to achieving a tension free anastomosis with well vascularised stapled ends? We present a series of consecutive AR undertaken by a single surgeon using a standardized technique which incorporates our routine use of ‘double verification of vascularity’ but with no ALT and assessed the resulting anastomotic leak rate as the endpoint.
We present the following article/case in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ales-20-97).
Methods
A retrospective analysis of a prospectively maintained database of consecutive patients who had undergone colorectal resections during a 15-year period [2004–2019] was undertaken. Only anterior resections with stapled anastomosis were assessed. We excluded any patients who required a concomitant resection of a solid viscus during the AR. Only patients >18 years of age were included in our cohort. We obtained our local hospital institutional review board’s permission to undertake this observational study. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). Because of the retrospective and observational nature of the study the requirement for informed consent was waived (2).
Data extracted included demography [age, sex, American Society of Anesthesiologists (ASA)], technique used (laparoscopic, open), timing (elective, emergency) and length of stay (LOS). The indication for the operation was documented. The type of AR was defined by the site of the distal transection and anastomosis: high if it is at more than 8 cm from the anal verge, low if at 5–8 cm from anal verge and ultra-low if at 3–5 cm from the anal verge.
We documented cases of anastomotic leak which is our end point. Leak was defined and graded (A, B, C) according to the International Study Group of Rectal Cancer (ISGRC) (3). The timing of leak was defined as peri-operative leak and delayed leak (≥6 weeks postoperative). The type of intervention required (if any) was recorded in case of leak.
Malignant cases were discussed in a gastrointestinal multidisciplinary meeting where a decision of upfront surgery or neoadjuvant therapy for rectal cancers was decided after consensus between surgeons and medical/radiation oncologist as per the local Colorectal cancer guidelines from the National Health and Medical Research Council (NHMRC) (4). All patients were assessed and optimised by the anaesthetic team. The operations were performed in Bankstown-Lidcombe or Waratah Private Hospitals (Sydney, Australia), which are both equipped with high dependency units to monitor high risk patients.
The operations were carried out in a standardized fashion by the senior author (C.R. Berney) who has extensive experience with advanced laparoscopic gastrointestinal procedures, including colorectal resections (5). Following routine pre-operative bowel preparation, patients were placed in lithotomy position with temporary nasogastric tube (NGT), indwelling catheter (IDC) and calf compressors. Combined 1 g of ceftriaxone and 500 mg of metronidazole were given intravenously at induction along with subcutaneous 20 mg of enoxaparin. A standard midline laparotomy was performed for open abdominal procedures, and a 4 ports approach was adopted for laparoscopic cases. A lateral to medial approach was favoured. The sigmoid and left colon were mobilised using Harmonic scalpel along its embryological plane, taking care to preserve the left ureter and gonadal vessels. The splenic flexure was routinely fully mobilised. The inferior mesenteric vessels were taken proximally only in malignant cases. Laparoscopically, those vessels were preferentially divided with 45 mm articulated Endo-GIATM vascular stapler (Echelon FlexTM, Ethicon, San Angelo, TX, USA). Dissection was extended in the presacral plane. The lateral ligaments were taken and retrovesical plane defined depending on the location of the rectal tumours. The rectum was divided with size 45 mm endo-GIATM (preferentially blue cartridges) staplers (Echelon FlexTM, Ethicon). In laparoscopic cases, the sigmoid and stapled proximal rectum were exteriorised via a left-sided muscle-splitting incision to minimize the risk of incisional hernia (if taken at the midline). An assessment of vascularity was made as per the serosal colour and mesenteric pulsation before proximal colonic transection was undertaken with a blade, after a 45 mm purse string device (PurstringTM AutosutureTM, Medtronic, Minneapolis, MN, USA) was applied. The transected colonic edges and luminal mucosa were inspected for vascularity. So, in effect there are two instances where vascularization can be demonstrated (‘double verification of vascularisation’). In case of doubt regarding viability the proximal colon was further divided at either of those checkpoints.
The purse-string situated at the proximal colonic end was further secured with four stay 3/0 PDS sutures, the anvil was introduced, and the purse-string tied. The choice of stapler head size and cartridge was made according to thickness of tissues and anorectal anatomy. An end-to-end anastomosis was generally fashioned using a size 29 mm EEATM (Medtronic) or ILS (Ethicon) circular stapler following rectal stump washout. The stapling was performed by a senior member of the team, taking care to strictly follow manufacturer’s instructions. Both donuts were carefully inspected for full thickness completeness. In case of doubt the anastomosis was redone. After ensuring a tension-free anastomosis and pelvic washout a Jackson Pratt drain was deployed in the pelvis and wounds closed. The NGT was immediately removed. Post operatively the patients were allowed clear fluids on the first day and gradually upgraded to normal diet, generally on the 2nd or 3rd day if well. Patient controlled analgesia was given for 2 days at most. IDC was taken down the next day and drain removed after patients had bowel motions.
After discharge all patients were routinely followed up by the senior author (C.R. Berney), initially at 2 and 6 weeks postoperative and then 3, 6 and 12 months. Patients were reminded to attend the clinic should they fail to do so in the prescribed timeframe. In case of any major postoperative issues the patients were instructed to contact the surgeon and present to Bankstown-Lidcombe hospital for further evaluation. All malignant cases were discussed in the multidisciplinary oncology meeting and follow up investigations undertaken according to the NHMRC guidelines (4).
Statistical analysis
Continuous variables were expressed as median and categorical variables were presented as absolute numbers or median.
Results
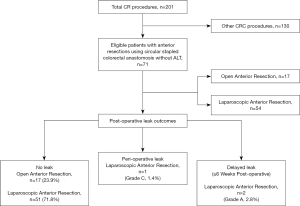
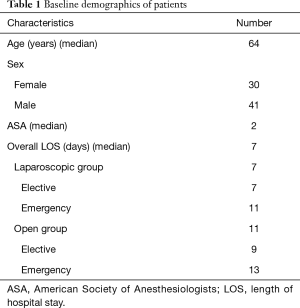
Out of 201 colorectal patients analysed during this 15-year period, 71 (35.3%) underwent ARs with end-to-end stapled anastomosis. This procedure was performed open in 17 cases (23.9%) and laparoscopically in the remaining 54 (76.1%) patients. Demographic characteristics of the patients and LOS details are presented in Table 1. One patient who required a simultaneous partial nephrectomy was excluded from the study.
Full table
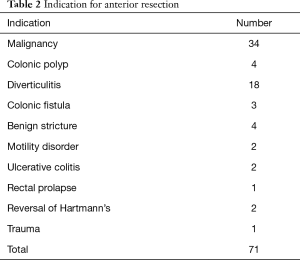
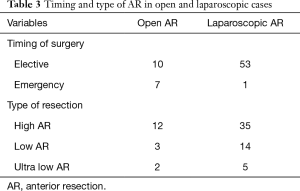
Table 2 details the indications for ARs. Thirty-four (47.9%) of our cohort patients had a preoperative diagnosis of adenocarcinoma, which was confirmed on histopathology. Four cases of rectal cancers received neoadjuvant radiotherapy and had defunctioning ileostomy. This is the only group of patients in our cohort who received an ileostomy. Forty-seven patients (66.2%) underwent a high-AR, 17 (23.9%) had a low-AR and 7 (9.9%) ultra-low-AR (Table 3).
Full table
Full table
There was only one post-operative leak (grade C) noted in our cohort, giving a rate of significant leak of 1.4% (Figure 1). This was diagnosed on day 4 post-surgery in a 40-year-old male who underwent laparoscopic high-AR for recurrent severe diverticulitis. This was managed by laparoscopic pelvic washout and drainage without the need for a stoma formation. The patient made an uneventful recovery. The enterococcus cultured from the exudate was resistant to ceftriaxone and metronidazole.
Two minor subclinical anastomotic-vaginal fistulae (grade A leaks; 2.8%) with no radiological or endoscopic evidence of leak were found after 6 weeks post-surgery and following laparoscopic low anterior resection for rectal cancer (no neoadjuvant therapy). This occurred in two female patients who had previously undergone hysterectomies. Both were successfully managed non-operatively as outpatients, with oral antibiotics and a low residual diet, with spontaneous closure of the fistulae and no clinical sequalae. There was no procedure related mortality noted in our series.
Discussion
Our overall anastomotic leak rate of 4.2% [one postoperative (1.4%) and two delayed minor leaks (2.8%)] compares favourably with ranges of published leak rates of 3–23% post anterior resection (1,6-9), despite not performing ALT and all those procedures being performed under the care of a “low-volume” colorectal surgeon. Our anastomotic vaginal fistula rate of 2.8% is comparable to recent series (10) although in our case those complications were only marginally significant, as both patients did not require intervention.
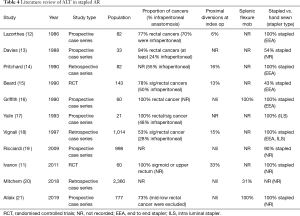
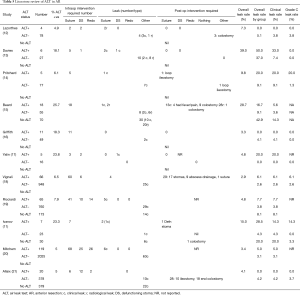
Over the last three decades the ALT has continued to be widely used as a means of intraoperative interrogation of a stapled anastomosis after AR, although only one very recent randomised study has demonstrated a benefit of the ALT (11). Is there enough strong evidence to suggest that the ALT provides adequate assessment of the stapled anastomosis? Does the result of the ALT dictate the risk of a postoperative leak? In order to answer those questions, we performed a literature review using PubMed and Medline to identify all studies that documented the use of ALT in AR. We selected 11 articles as detailed in Tables 4,5.
Full table
Full table
A number of confounders that are likely to affect the rate of postoperative leak rate were identified in the various studies. The indication for the AR was either not clearly delineated or very variable: three studies (14,19,20) did not report the proportion of cancers in their cohort and the proportion of patients with rectal/sigmoid cancers in the remaining studies varied from 53% to 100%. Among those series that described the location of the rectal cancers, the proportion of infra-peritoneal lesions was again variable and was as high as 70% (12). In addition, the use of neoadjuvant therapy was not declared or described in series, which included low rectal cancers. The rate of proximal diversion varied between 0% and 33% (11,12,14-16,18-21). Also, only three studies (16,20,21) quantified the number of patients who had splenic flexure mobilisation.
The anastomotic technique was also not consistent across the studies. The EEA stapler was the commonest stapling device used, but 5 series did not declare the type used. Three studies (13,15,19) included hand-sewn anastomosis in their series. This is important as the method of anastomosis can influence the leak rate. Thus, the most contemporary series (19) in our review to contain a combination of stapled and hand-sewn anastomosis showed a clinical leak rate of 4.4% in the stapled group and 8.1% in the hand-sewn group.
It is also important to carefully assess the way ALT has been validated. A recent meta-analysis (22) showed a high risk of bias in studies assessing ALT as a way of anastomotic interrogation. As evidenced by our literature review, there are issues with the quality of the studies: there are only 2 randomised controlled trials published, only 4 studies included controls, 7 of the 11 studies included less than 100 patients. We note that the technique of ALT was not standardized. Importantly, the definition of the leak was variable across studies and not standardized as per ISGRC. Four studies (15,17,19,20) did not even declare the rate of grade C leaks.
So, does an ALT really help reduce the postoperative leak rate after stapled anastomosis? We identified 6 studies (11-14,16,18) that allowed direct assessment of the potential benefit of ALT on reducing both, the radiological and/or clinical incidence of grade C leaks (Figures 2,3). Only one of the 6 studies was randomised (11). The total leak rate varied widely between 0% and 50% in the ALT+ cases even though, in those situations, the defect was apparently repaired intraoperatively. Most importantly, ALT- groups still reported postoperative leak rates ranging from 2.6% to 37%, even though the anastomosis should have been airtight. Although five of the six studies showed a reduction in corresponding grade C leaks, 3 series (11,18,23) still reported leak rates of 6.1% to 20%. Interestingly they were from ALT+ groups.
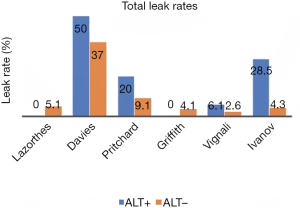
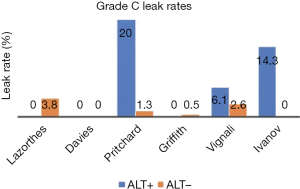
How do we explain persistent postoperative leak rate despite ALT being performed? The leaks in ALT− groups could have been false negatives, for instance due to lack of rigour or standardization of technique (low volume of insufflation, inadequate proximal bowel clamping). Importantly, the higher leak rate in ALT+ cases might be partly due to bias: it is possible that surgeons undertook ALT in the anastomosis which they thought were more at risk of leak. Another possible explanation is that ALT may adversely affect the newly fashioned anastomosis. Indeed, it has been shown that a luminal pressure of at least 25 to 30 cmH2O is required to demonstrate the airtightness of the anastomosis (24). Such pressure, especially if not standardised or monitored, might cause direct barotrauma to the anastomosis, especially one which is at higher risk by virtue of low rectal anastomosis or neoadjuvant radiotherapy (25,26).
There was heterogeneity in the technique used to deal with positive ALTs across some of the 11 studies assessed. We only included those that declared the correlation between grade C leaks and the technique initially used to repair it (suturing, defunctioning proximal stoma, redo anastomosis). Three studies only used suturing as the preferred technique to manage positive ALT (Table 6). Four studies adopted a multimodal approach to controlling those ALT+ cases (Table 7), depending on the location, magnitude of the leak and the experience of the surgeon. It is interesting to note that when suturing was used as the only technique to deal with air leaks, there were still some grade C leaks subsequently noted. However, when a multimodal approach was adopted there were no grade C leaks post operatively, except in one study where the suture group outcome was not fully reported (19).

Full table
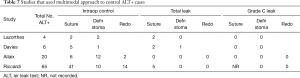
Full table
It is important to remember that the technique of the stapled anastomosis may also affect the risk of leak, irrespective of the ALT finding. All of our stapled anastomoses were achieved with a carefully chosen stapling device after sizing the rectum and defining the quality and vascularity of the tissues. A senior member of the surgical team always deployed the stapling devices. Indeed, the technique of deployment has been shown to be equally as important in order to prevent a poorly constructed anastomosis. Offodile (27) has shown that anastomotic complications can be attributed to lack of experience of the “deployer”. The resistance and ‘abnormal feel’ during stapler deployment require experience that the participating registrars in theatre may lack (28). Moreover, the experience of the surgeon is key in deciding on the perfect fit of the stapler/cartridge combination for that particular patient. The decision may be also influenced by neoadjuvant radiotherapy which affects tissue compressibility and hence the best type of cartridge to be used.
There is currently no robust guideline from any international colorectal associations to strongly promote ALT. A collaboration of colorectal surgeons who met in London in 2016 cast some doubt on the validity of the ALT in the light of a recent high volume controlled study (19). One of the strengths of our technique is the use of the ‘double verification of vascularity’ of the proximal colon before constructing the anastomosis. This is especially important during the laparoscopic approach, which is our favoured technique. In the first instance exteriorisation of the colon after rectal transection allows us to note any vascular demarcation on the serosal surface and also palpate the mesenteric arterial pulse before undertaking the colonic transection. This enables us to choose the safest place for transection. Secondly, we verify the vascularity of the luminal mucosa end of the transected edge of the colon at the time of insertion of the anvil. This internal inspection is possible due to our use of the purse-string device. The colon could be further resected at either of those two check points. A pure laparoscopic approach would not always allow this ‘double verification of vascularity’ and hence may possibly allow construction of a compromised anastomosis.
Another important technical adjustment that could potentially reduce the risk of grade C leak is that for every purse-string applied at the proximal colonic end, we further reinforce it with four corners full thickness 3/0 PDS sutures before introducing the anvil. That way, the purse-string can be securely tied around the tip of the anvil without risking tearing off the mucosa edges. Finally, we also routinely inspect integrity of both donuts and always make certain that the colorectal anastomosis is under no tension by full mobilisation of the splenic flexure.
Our study has a number of deficiencies with respect to assessment of leak rate and assessing the role of ALT. We did not have a comparison arm, which could have been obtained by including cases from other surgeons in the unit who routinely perform ALT, but that would have introduced other confounders (29-36). Moreover, our caseload is not homogeneous. We included benign and malignant cases, which has implication in extent of dissection, rate of IMA sparing approaches and hence may influence degree of ischaemia and postoperative leak. Of importance is that this is a retrospective analysis of a prospectively maintained database of consecutive unselected patients who had undergone colorectal resections and therefore more representative of what a general surgeon with “low-volume” colorectal activity would normally be dealing with. Interestingly, we were still able to reproduce excellent results in term of postoperative leak, when compared to other “high-volume” colorectal groups.
Conclusions
Whilst an ALT can be easily undertaken after a stapled AR, the surgeon should be cautious in its interpretation. Firstly, it should not be used to verify soundness of a poorly constructed anastomosis. Secondly, a negative ALT does not always mean the anastomosis will not leak. More attention needs to be paid to the tenets of a sound stapled anastomosis after an AR: adequate colonic mobilisation to ensure a tension free anastomosis, well vascularised stapled ends and proper deployment of an appropriately sized endoanal stapler with a well-selected cartridge. Thus, the surgeon’s skills, judgement and experience may obviate the need for a test that still needs full validation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ales-20-97
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ales-20-97
Peer Review File: Available at http://dx.doi.org/10.21037/ales-20-97
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-97). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. We obtained our local hospital institutional review board’s permission to undertake this observational study. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). Because of the retrospective and observational nature of the study the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Issues in Professional Practice: Prevention, diagnosis and management of colorectal anastomotic leakage. March 2016. Association of Surgeons of Great Britain and Ireland.
- National Statement on Ethical Conduct in Human Research 2007 (Updated 2018). The National Health and Medical Research Council, the Australian Research Council and Universities Australia. Commonwealth of Australia, Canberra.
- Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010;147:339-51. [Crossref] [PubMed]
- Cancer Council Australia Colorectal Cancer Guidelines Working Party. Clinical practice guidelines for the prevention, early detection and management of colorectal cancer. Sydney: Cancer Council Australia.
- Gandy RC, Berney CR. Safety of laparoscopic colorectal surgery in a low-volume setting: review of early and late outcome. Gastroenterol Res Pract 2014;2014:581523. [Crossref] [PubMed]
- Rullier E, Laurent C, Garrelon JL, et al. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg 1998;85:355-8. [Crossref] [PubMed]
- Hüser N, Michalski CW, Erkan M, et al. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg 2008;248:52-60. [Crossref] [PubMed]
- Hirst NA, Tiernan JP, Millner PA, et al. Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Colorectal Dis 2014;16:95-109. [Crossref] [PubMed]
- Dickson GH. Intraoperative testing of the integrity of left-sided colorectal anastomoses: a technique of value to the surgeon in training. Ann R Coll Surg Engl 1990;72:66. [PubMed]
- Matthiessen P, Hansson L, Sjödahl R, et al. Anastomotic-vaginal fistula (AVF) after anterior resection of the rectum for cancer--occurrence and risk factors. Colorectal Dis 2010;12:351-7. [Crossref] [PubMed]
- Ivanov D, Cvijanovic R, Gvozdenovic L. Intraoperative air testing of colorectal anastomoses. Srp Arh Celok Lek 2011;139:333-8. [Crossref] [PubMed]
- Lazorthes F, Chiotassol P. Stapled colorectal anastomoses: peroperative integrity of the anastomosis and risk of postoperative leakage. Int J Colorectal Dis 1986;1:96-8. [Crossref] [PubMed]
- Davies AH, Bartolo DC, Richards AE, et al. Intra-operative air testing: an audit on rectal anastomosis. Ann R Coll Surg Engl 1988;70:345-7. [PubMed]
- Pritchard GA, Krouma FF, Stamatakis JD. Intraoperative testing of colorectal anastomosis can be misleading. Br J Surg 1990;77:1105. [Crossref] [PubMed]
- Beard JD, Nicholson ML, Sayers RD, et al. Intraoperative air testing of colorectal anastomoses: a prospective, randomized trial. Br J Surg 1990;77:1095-7. [Crossref] [PubMed]
- Griffith CD, Hardcastle JD. Intraoperative testing of anastomotic integrity after stapled anterior resection for cancer. J R Coll Surg Edinb 1990;35:106-8. [PubMed]
- Yalin R, Aktan AO, Yeğen C, et al. Importance of testing stapled rectal anastomoses with air. Eur J Surg 1993;159:49-51. [PubMed]
- Vignali A, Fazio VW, Lavery IC, et al. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1, 014 patients. J Am Coll Surg 1997;185:105-13. [Crossref] [PubMed]
- Ricciardi R, Roberts PL, Marcello PW, et al. Anastomotic leak testing after colorectal resection: what are the data? Arch Surg 2009;144:407-11; discussion 411-2. [Crossref] [PubMed]
- Mitchem JB, Stafford C, Francone TD, et al. What is the optimal management of an intra-operative air leak in a colorectal anastomosis? Colorectal Dis 2018;20:O39-O45. [Crossref] [PubMed]
- Allaix ME, Lena A, Degiuli M, et al. Intraoperative air leak test reduces the rate of postoperative anastomotic leak: analysis of 777 laparoscopic left-sided colon resections. Surg Endosc 2019;33:1592-9. [Crossref] [PubMed]
- Wu Z, van de Haar RC, Sparreboom CL, et al. Is the intraoperative air leak test effective in the prevention of colorectal anastomotic leakage? A systematic review and meta-analysis. Int J Colorectal Dis 2016;31:1409-17. [Crossref] [PubMed]
- Pommergaard HC, Gessler B, Burcharth J, et al. Preoperative risk factors for anastomotic leakage after resection for colorectal cancer: A systematic review and meta-analysis. Colorectal Dis 2014;16:662-71. [Crossref] [PubMed]
- Smith S, McGeehin W, Kozol R, et al. The efficacy of intraoperative methylene blue enemas to assess the integrity of a colonic anastomosis. BMC Surg 2007;7:15. [Crossref] [PubMed]
- Pettersson D, Cedermark B, Holm T, et al. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg 2010;97:580-7. [Crossref] [PubMed]
- Matthiessen P, Hallbook O, Andersson M, et al. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis 2004;6:462-9. [Crossref] [PubMed]
- Offodile AC 2nd, Feingold DL, Nasar A, et al. High incidence of technical errors involving the EEA circular stapler: a single institution experience. J Am Coll Surg 2010;210:331-5. [Crossref] [PubMed]
- Sugarbaker PH. Rectovaginal fistula following low circular stapled anastomosis in women with rectal cancer. J Surg Oncol 1996;61:155-8. [Crossref] [PubMed]
- Lanthaler M, Biebl M, Mittermair R, et al. Intraoperative colonoscopy for anastomosis assessment in laparoscopically assisted left-sided colon resection: is it worthwhile? J Laparoendosc Adv Surg Tech A 2008;18:27-31. [Crossref] [PubMed]
- Li VK, Wexner SD, Pulido N, et al. Use of routine intraoperative endoscopy in elective laparoscopic colorectal surgery: can it further avoid anastomotic failure? Surg Endosc 2009;23:2459-65. [Crossref] [PubMed]
- Shamiyeh A, Szabo K, Ulf Wayand W, et al. Intraoperative endoscopy for the assessment of circular-stapled anastomosis in laparoscopic colon surgery. Surg Laparosc Endosc Percutan Tech 2012;22:65-7. [Crossref] [PubMed]
- Sujatha-Bhaskar S, Jafari MD, Hanna M, et al. An endoscopic mucosal grading system is predictive of leak in stapled rectal anastomoses. Surg Endosc 2018;32:1769-75. [Crossref] [PubMed]
- Jafari MD, Lee KH, Halabi WJ, et al. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 2013;27:3003-8. [Crossref] [PubMed]
- Kin C, Vo H, Welton L, et al. Equivocal effect of intraoperative fluorescence angiography on colorectal anastomotic leaks. Dis Colon Rectum 2015;58:582-7. [Crossref] [PubMed]
- Kim JC, Lee JL, Yoon YS, et al. Utility of indocyanine-green fluorescent imaging during robot-assisted sphincter-saving surgery on rectal cancer patients. Int J Med Robot 2016;12:710-7. [Crossref] [PubMed]
- Vallance A, Wexner S, Berho M, et al. A collaborative review of the current concepts and challenges of anastomotic leaks in colorectal surgery. Colorectal Dis 2017;19:O1-O12. [Crossref] [PubMed]
Cite this article as: Rajkomar K, Moollan Y, Badiani S, Zhao C, Berney CR. Is air leak testing really necessary after stapled anterior resection?—a case series and a literature review. Ann Laparosc Endosc Surg 2021;6:4.