Minimally invasive proximal gastrectomy and double tract reconstruction
Introduction
Whereas gastric cancer in general is on the decline, proximal gastric cancer and cancer of the esophagogastric junction in specific is rising in incidence (1,2). This tumor location has been a surgical challenge ever since and until today the optimal treatment is debated. On the one hand, for cancer of the esophagogastric junction, esophagectomy or gastrectomy are routinely performed strategies, depending on the exact localization of the tumor (3,4). On the other hand, if gastrectomy is performed or in proximal gastric cancer, proximal gastrectomy with preservation of the distal stomach can be performed. The proximal gastrectomy is not only a question of oncological safety in regards of radicality of the surgery, but also a question of reconstruction in order to turn the distal stomach preservation into a functional benefit for the patient. Several different reconstruction types are known after proximal gastrectomy, but none has reached to be routinely performed, which is due to the controversies of the resection type but also due to the diverse outcomes and complexity of the reconstruction (5).
Lately, another reconstruction type, the double tract method, became more popular, especially in Korea, where a prospective randomized trial is evaluating the benefits in comparison to total gastrectomy in early proximal gastric cancer (6,7). In Europe, very few experiences exist, but undoubtedly any reconstruction after proximal gastrectomy is of higher interest, which motivates to present the current status of this relatively new technique. A PubMed and Medline based research without certain period including the keywords “proximal gastrectomy” and “double tract reconstruction” was performed and eligible publications were used. This review comprises the features of proximal gastrectomy with double tract reconstruction and advances possible ways of application western countries.
Proximal gastrectomy
Proximal gastric cancers or cancers of the esophagogastric junction, which are applicable via gastrectomy, are often resectable with a proximal gastrectomy in a transabdominal approach with or without resection of the distal esophagus (4). The luminal extent of resection mainly refers to certain desired safety margins, wherefore different recommendations can be found in national and international guidelines (8-11). As the mentioned tumor locations mostly determine the proximal, esophageal margin to be the one at higher risk, proximal gastrectomy might be feasible in quite a number of cases instead of total gastrectomy in regards of the radicality of the luminal resection. However, the oncological radicality of the surgery also mainly depends on the lymphadenectomy, which is somehow connected to the principle of stomach preservation. In case of proximal gastrectomy, in order to maintain optimal blood supply to the remnant distal stomach, usually parts of the lesser curvature (lymph node station 3b and 5) as well as the greater curvature (lymph node station 4d and 6) are (partly) preserved (10). In this situation, blood supply is maintained through right gastric artery and right gastroepiploic artery, including the infrapyloric vessels. As these mentioned Lymph node stations belong to the D1 compartment of lymphadenectomy in gastric cancer, proximal gastrectomy finds it general recommendation in cases of early cancer. In Japan and Korea, proximal gastrectomy is more routinely performed as to the high incidence of early cancers (clinically T1 category). In European countries, early gastric cancers at time of diagnosis are rare, but paradigms of lymphadenectomy are not interpretated as irrevocably and advances in multimodal approaches would more often lead to complete response at time of surgery, which requires reevaluation of the standard surgical approach.
Reconstruction: the double tract method
After decision is made to perform a proximal gastrectomy, choice also has to be made concerning the following reconstruction. Several reconstruction types have been described for proximal gastrectomy. The most direct way, performing an esophagogastrostomy, comes naturally along with severe reflux issues (12,13). Ways of performing the esophagogastrostomy with anti-reflux flap, the double flap method, is mainly performed in Japan. This reconstruction type requires both, a very limited gastric resection and a decent case load of gastric cancer resections in order to perform this method successfully, as it is technically very challenging (13,14).
The jejunal interposition or Merendino operation, has been discussed as an option for the cardia resection in benign indications as well as in early cancers but is also left controversial regarding its functional outcome (15). Redundancy of the jejunal limb can lead to severe problems of the food passage, ending up in correctional surgery in some cases. Reflux symptoms are still present in a relevant number of patients. Some alternations of the Merendino operation have been described, but none would lead to routine use or clear superiority in outcomes.
The double tract reconstruction after proximal gastrectomy have been firstly described in 1988 in Japan (16). As far as the literature tells, it was not widely performed until the rising incidence of proximal gastric cancer combined with screening programs for early gastric cancer detection in Japan and Korea put the limited proximal resection in the focus.
The Korean LAparoendoscopic gastrointestinal Surgery Study (KLASS) Group has initiated a prospective randomized trial (KLASS-05) in order to compare the proximal gastrectomy with double tract reconstruction with the total gastrectomy for early gastric cancer of the upper third (6,7). Primary endpoint of this first prospective trial for this reconstructions type is the hemoglobin level after 3 years of follow-up. Previous retrospective studies have indicated an improved performance of patients undergoing double tract in regards of Quality of Life and postoperative development of Hemoglobin levels. It is assumed that the distal stomach preservation contributes to higher levels of Vitamin B12, which reduces the postoperative anemia. These interferences are based on retrospective data mainly firstly from Korea but also from Japan and China and are pending to be confirmed either by the prospective trial KLASS-05 or prospective studies from other countries (17-20).
The only Western publication on the double tract reconstruction is from Germany and introduces this method as alternative to jejunal interposition (Merendino) or total gastrectomy (21). The authors conclude that referring to the oncological radicality no difference is expected in comparison to reconstruction with jejunal interposition and in respect of the appropriate indication no difference to total gastrectomy. Solid data about direct comparison of jejunal interposition and double tract reconstruction is however pending.
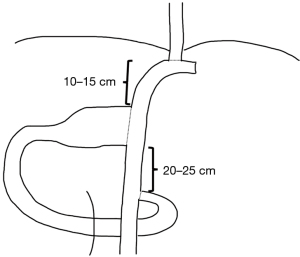
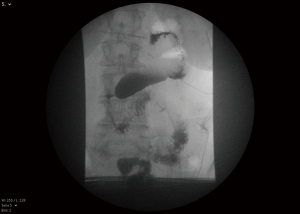
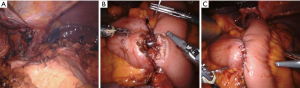
Regarding the technical aspects of the double tract method, minimally invasive surgery finds a prominent role in the recent descriptions and studies (20,22). As early cancer represents the classical indication for proximal gastrectomy, in east Asia laparoscopic surgery and robotic is routinely performed (23). The reconstruction in double tract procedure can be performed partly extracorporeal through mini laparotomy after retrieving the specimen an includes three anastomoses, an esophagojejunostomy, gastrojejunostomy and jejunojejunostomy (Figure 1). A published method from Korea includes extracorporeal performance of the jejuno-jejunostomy and of the gastrojejunostomy and intracorporeal performance of the esophagojejunostomy. Ahn et al. describe a 10-cm distance between the esophagojejunostomy and gastrojejunostomy and a 20-cm distance between the gastrojejunostomy and jejunostomy (19). Hölscher et al. mention 15 cm and 25 cm respectively and possibly the larger distanced contribute to a satisfactory outcome in Western patience with higher risk of reflux due to body constitution (21). The reconstruction can be performed both antecolic and retrocolic. Antecolic reconstruction appears more feasibly in minimally invasive surgery, differences regarding this particular reconstruction methods have not been studied. The esophagojejunostomy and the jejunojejunostomy can be performed similarly as in total gastrectomy. The gastrojejunostomy, if performed intracorporeally, can be done as hand-sewn anastomosis or linear stapler anastomosis. For hand-sewn technique, the robotic system seems beneficial. In this case, the stapler transection of the stomach can be partly resected, followed by a continuous single end-to-side gastrojejunostomy (Figure 2). In the laparoscopic setting, the gastrojejunostomy can be performed on the anterior or posterior side of the stomach as side-to-side gastrojejunostomy with hand-sewn closure of the stapler entrance. Postoperative abdominal X-ray can demonstrate the passage through both, stomach and jejunum (Figure 3). The interference of anastomotic technique of the gastrojejunostomy and the postoperative function of the stomach is not clear yet.
Discussion
Proximal gastric cancer and cancer of the esophagogastric junction remain surgical challenges due to perioperative safety as well as to postoperative functional outcome. Cancer of the esophagogastric junction, including AEG type I and II is also treatable via abdominothoracic esophagectomy and gastric pull-up, which is nower days more and more performed minimally invasive or robotic-assisted (3,24-27). In case of gastrectomy, for AEG Type I and II and proximal gastric cancer, an organ preservation is possible by leaving a distal remnant. Several reconstruction methods after proximal gastrectomy have been published, but none would have let to such satisfactory results to establish a widely accepted routine procedure (13). The double tract reconstruction is one of the methods to have gained more popularity lately. A Korean prospective randomized trial (KLASS-05) is about to evaluate functional benefits over total gastrectomy for early proximal gastric cancer (6).
A clear oncological indication for proximal gastrectomy is given in case of early (cT1) gastric or AEG Type II/III cancer, which is commonly only found in countries with successful screening programs like Japan or Korea. In most western countries, early gastric cancer detection remains a seldom event. But it is not yet systematically investigated, if proximal gastrectomy is oncologically safe in more advanced cancers, especially after major or complete response after neoadjuvant treatment. Regarding this question, Italian multicenter retrospective data and data from Japan from T2/T3 cancers agree that no survival difference is to be expected from proximal gastrectomy compared to total gastrectomy (12,28). The luminal extent of resection is determined by the dimension of the tumor and the ultimate goal is to achieve R0 resection. Recent results indicate that in R0 resection of gastric cancer, the extent of safety margin does not affect survival (29). The chance of R0 resection is maximized if a minimum of 3 cm macroscopic margin and a negative frozen section is secured intraoperatively. The compromise in lymphadenectomy in proximal gastrectomy compared to total gastrectomy mainly affects the infrapyloric area and the right gastric artery, whereas the whole D2 level can be dissected completely (21). The lymph node harvest around the right gastric artery is minimal, however it can be sacrificed without consecutive necrosis of the remnant distal stomach. One method for additional oncological safety might be to dissect representative lymph nodes from the infrapyloric region for intraoperative frozen section investigation in order to confirm that no signs of cancer infiltration are seen. Interestingly, vessel preserving complete lymphadenectomy around the infrapyloric vessels is the key routine maneuver in pylorus preserving gastrectomy for early gastric cancer in Japan and Korea (30-32). Although this particular procedure is even less commonly performed than the proximal gastrectomy in western countries, it might give important implications for extension of indication for proximal gastrectomy. In addition to the controversies of indication, it also remains unclear, what size of distal remnant minimally needs to be preserved in order to obtain functional benefits for the patient in comparison to total gastrectomy. After showing the feasibility of the reconstruction method in the West, it clearly needs prospective trials to evaluate the functional benefits over total gastrectomy.
Proximal gastrectomy plus double tract reconstruction is feasible to be performed via minimally invasive surgery. As different trials suggested equal survival of laparoscopic and open gastrectomy in advanced gastric cancer, it is expected to become also a routine method in western countries, at least in specialized centers (33,34).
Conclusions
Proximal gastrectomy with double tract reconstruction gains more popularity recently and might represent a worthy alternative option to total gastrectomy in proximal gastric cancer or cancer of the esophagogastric junction. As first studies suggest functional benefits of this reconstruction method it urgently needs to be prospectively evaluated for its oncological safety. The procedure qualifies for minimally invasive surgery and therefor fits in the future routine concepts of gastric and junctional cancer surgical treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Stefano Rausei and Simone Giacopuzzi) for the series “Minimally Invasive Surgery and Gastric Cancer: Where Are We Now?” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-21-13/coif). The series “Minimally Invasive Surgery and Gastric Cancer: Where Are We Now?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deans C, Yeo MS, Soe MY, et al. Cancer of the gastric cardia is rising in incidence in an Asian population and is associated with adverse outcome. World J Surg 2011;35:617-24. [Crossref] [PubMed]
- Steevens J, Botterweck AA, Dirx MJ, et al. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur J Gastroenterol Hepatol 2010;22:669-78. [PubMed]
- Berlth F, Hoelscher AH. History of Esophagogastric Junction Cancer Treatment and Current Surgical Management in Western Countries. J Gastric Cancer 2019;19:139-47. [Crossref] [PubMed]
- Rüdiger Siewert J, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 2000;232:353-61. [Crossref] [PubMed]
- Ryu KW, Park YS, Kwon OK, et al. Korean practice guideline for gastric cancer 2018: An evidence-based, multi-disciplinary approach. J Gastric Cancer 2019;19:1-48. Erratum in: J Gastric Cancer 2019;19:372-3. [Crossref] [PubMed]
- Park DJ, Park YS, Ahn SH, et al. Laparoscopic Proximal Gastrectomy as a Surgical Treatment for Upper Third Early Gastric Cancer. Korean J Gastroenterol 2017;70:134-40. [Crossref] [PubMed]
- Berlth F, Yang HK. Minimal-invasive gastrectomy: what the west can learn from the east? Updates Surg 2018;70:181-7. [Crossref] [PubMed]
- Meyer HJ, Hölscher AH, Lordick F, et al. Current S3 guidelines on surgical treatment of gastric carcinoma. Chirurg 2012;83:31-7. [Crossref] [PubMed]
- Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi57-63. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- De Manzoni G, Marrelli D, Baiocchi GL, et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer 2017;20:20-30. [Crossref] [PubMed]
- Rosa F, Quero G, Fiorillo C, et al. Total vs proximal gastrectomy for adenocarcinoma of the upper third of the stomach: a propensity-score-matched analysis of a multicenter western experience (On behalf of the Italian Research Group for Gastric Cancer-GIRCG). Gastric Cancer 2018;21:845-52. [Crossref] [PubMed]
- Nunobe S, Ida S. Current status of proximal gastrectomy for gastric and esophagogastric junctional cancer: A review. Ann Gastroenterol Surg 2020;4:498-504. [Crossref] [PubMed]
- Mine S, Nunobe S, Watanabe M. A Novel Technique of Anti-reflux Esophagogastrostomy Following Left Thoracoabdominal Esophagectomy for Carcinoma of the Esophagogastric Junction. World J Surg 2015;39:2359-61. [Crossref] [PubMed]
- Merendino KA, Dillard DH. The concept of sphincter substitution by an interposed jejunal segment for anatomic and physiologic abnormalities at the esophagogastric junction; with special reference to reflux esophagitis, cardiospasm and esophageal varices. Ann Surg 1955;142:486-506. [Crossref] [PubMed]
- Aikou T, Natsugoe S, Shimazu H, et al. Antrum preserving double tract method for reconstruction following proximal gastrectomy. Jpn J Surg 1988;18:114-5. [Crossref] [PubMed]
- Ko HJ, Kim KH, Lee SH, et al. Can Proximal Gastrectomy with Double-Tract Reconstruction Replace Total Gastrectomy? A Propensity Score Matching Analysis. J Gastrointest Surg 2020;24:516-24. [Crossref] [PubMed]
- Yamashita K, Iwatsuki M, Koga Y, et al. Preservation of physiological passage through the remnant stomach prevents postoperative malnutrition after proximal gastrectomy with double tract reconstruction. Surg Today 2019;49:748-54. [Crossref] [PubMed]
- Ahn SH, Jung DH, Son SY, et al. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer 2014;17:562-70. [Crossref] [PubMed]
- Jung DH, Lee Y, Kim DW, et al. Laparoscopic proximal gastrectomy with double tract reconstruction is superior to laparoscopic total gastrectomy for proximal early gastric cancer. Surg Endosc 2017;31:3961-9. [Crossref] [PubMed]
- Hölscher A, Berlth F, Hemmerich M, et al. Double-Tract-Rekonstruktion nach Resektion des gastroösophagealen Übergangs wegen AEG-II- und -III-Tumoren Zentralbl Chir 2020;145:35-40. [Double Tract Reconstruction Following Limited Oesophagogastric Resection of AEG Types II and III Adenocarcinomas]. [Crossref] [PubMed]
- Lee CM, Park DW, Jung DH, et al. Single-Port Laparoscopic Proximal Gastrectomy with Double Tract Reconstruction for Early Gastric Cancer: Report of a Case. J Gastric Cancer 2016;16:200-6. [Crossref] [PubMed]
- Kim HI, Han SU, Yang HK, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg 2016;263:103-9. [Crossref] [PubMed]
- Siewert JR, Hölscher AH, Becker K, et al. Cardia cancer: attempt at a therapeutically relevant classification. Chirurg 1987;58:25-32. [PubMed]
- Grimminger PP, Hadzijusufovic E, Lang H. Robotic-Assisted Ivor Lewis Esophagectomy (RAMIE) with a Standardized Intrathoracic Circular End-to-side Stapled Anastomosis and a Team of Two (Surgeon and Assistant Only). Thorac Cardiovasc Surg 2018;66:404-6. [Crossref] [PubMed]
- Grimminger PP, Tagkalos E, Hadzijusufovic E, et al. Change from Hybrid to Fully Minimally Invasive and Robotic Esophagectomy is Possible without Compromises. Thorac Cardiovasc Surg 2019;67:589-96. [Crossref] [PubMed]
- Tagkalos E, Goense L, Hoppe-Lotichius M, et al. Robot-assisted minimally invasive esophagectomy (RAMIE) compared to conventional minimally invasive esophagectomy (MIE) for esophageal cancer: a propensity-matched analysis. Dis Esophagus 2020; [Crossref] [PubMed]
- Yura M, Yoshikawa T, Otsuki S, et al. Oncological safety of proximal gastrectomy for T2/T3 proximal gastric cancer. Gastric Cancer 2019;22:1029-35. [Crossref] [PubMed]
- Berlth F, Kim WH, Choi JH, et al. Prognostic Impact of Frozen Section Investigation and Extent of Proximal Safety Margin in Gastric Cancer Resection. Ann Surg 2020;272:871-8. [Crossref] [PubMed]
- Haruta S, Shinohara H, Ueno M, et al. Anatomical considerations of the infrapyloric artery and its associated lymph nodes during laparoscopic gastric cancer surgery. Gastric Cancer 2015;18:876-80. [Crossref] [PubMed]
- Park DJ, Lee HJ, Jung HC, et al. Clinical outcome of pylorus-preserving gastrectomy in gastric cancer in comparison with conventional distal gastrectomy with Billroth I anastomosis. World J Surg 2008;32:1029-36. [Crossref] [PubMed]
- Hiki N, Sano T, Fukunaga T, et al. Survival benefit of pylorus-preserving gastrectomy in early gastric cancer. J Am Coll Surg 2009;209:297-301. [Crossref] [PubMed]
- Yu J, Huang C, Sun Y, et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA 2019;321:1983-92. [Crossref] [PubMed]
- Hyung WJ, Yang HK, Park YK, et al. Long-Term Outcomes of Laparoscopic Distal Gastrectomy for Locally Advanced Gastric Cancer: The KLASS-02-RCT Randomized Clinical Trial. J Clin Oncol 2020;38:3304-13. [Crossref] [PubMed]
Cite this article as: Berlth F, Tagkalos E, Mann C, Hadzijusufovic E, Hölscher AH, Lang H, Grimminger PP. Minimally invasive proximal gastrectomy and double tract reconstruction. Ann Laparosc Endosc Surg 2022;7:8.