
The role of sphincter repair for fecal incontinence
Fecal incontinence is a multifactorial condition and can be associated with a sphincter defect that is a result of obstetrical injury, trauma, surgical care, or other iatrogenic injury. Prior to sacral neuromodulation (SNM) being approved by the Food and Drug Administration (FDA) in March 2011 (1) and becoming popularized, sphincter repair was the most common surgery offered to women with fecal incontinence and an anterior sphincter defect (2). Unfortunately, the durability of sphincter repair when studied over time has shown a deterioration in function (3). Initially, published results for overlapping sphincteroplasty success rate in the treatment of fecal incontinence were very optimistic (4). Later data showed that with careful tools to assess patients and more in depth follow up, the short-term success varies between 50–86%. The functional success after redo sphincter repair surgery is much worse, with only 28% success. Long-term outcomes demonstrated poor results with no patients fully continent at ten-year follow up in a study from our institution(5). So recently, the role of sphincter repair have been under debate.
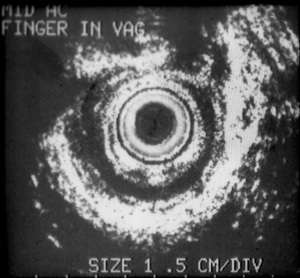
Nevertheless, the work-up for fecal incontinence always begins with a thorough history and physical exam. Anorectal physiology testing and endoanal ultrasound may help in determining treatment options. For unclear sphincter defects, an anal ultrasound may be beneficial to delineate the degree of the defect and involvement with internal and external sphincter (Figure 1). If a sphincter defect is identified on endoanal ultrasound or physical exam, then a sphincteroplasty could be considered in the algorithm of fecal incontinence treatment in selected cases. One such situation is treatment within the first year of a sphincter disruption from an obstetrical injury. Typically, the sphincter tear is associated with 3rd or 4th degree tear but may also be seen with lesser degree tears or episiotomy. Repair of an anterior sphincter defect with an overlapping sphincteroplasty may be performed three to six months after delivery, when the tissue is soft and supple with no inflammation or infection. When counseling a patient about a sphincter repair, it is important to set realistic expectations. At our institution these women are advised that the chance of needing further intervention is high within the first 5–10 years (5). In addition, discussing with the patients the advantages and disadvantages of all available options is important. When to perform the repair also is somewhat controversial. There has been literature regarding repair within the first month after an obstetrical injury (6). However our practice has been to allow this inflamed tissue to heal before attempting the secondary repair. Also, women with a cloacal defect from any cause are typically offered repair as an initial treatment when the tissue is soft and supple. A separate group of women have an anterior sphincter defect from childbirth but compensate for years before experiencing fecal incontinence. For these patients, a sphincter repair years after the obstetrical injury would not typically be offered due to the poor long-term results. A multipronged approach is initiated which includes nonsurgical treatment. Appropriate patients may then be offered sacral nerve stimulator (SNS).
Another area where sphincteroplasty is utilized is in conjunction with an anal or rectovaginal fistula (RVF) repair. These fistulae may or may not be associated with recent obstetrical injury. An epsio-proctotomy involves unroofing the RVF which in essence creates a defect that is like a 4th degree obstetrical injury. The overlap of the anterior sphincter defect then incorporates the fistula tract and closes the fistula.
In some countries, SNM is not available or the cost is prohibitive. For these women with fecal incontinence and an anterior sphincter defect, repair can be used as a primary treatment regardless of when the sphincter injury occurred. Additionally, after hearing all options for surgical intervention for fecal incontinence, some women prefer repair rather than an implantable device and for those women, sphincter repair can be offered with appropriate counseling regarding long-term outcomes.
When to do a diverting stoma at the time of an anal sphincter repair is also not well defined. There has not been evidence that diversion helps with improvement in repair. Our team considers a stoma if there has been an associated fistula, when sepsis cannot be controlled, when the surgery is a redo or there is extensive scarring.
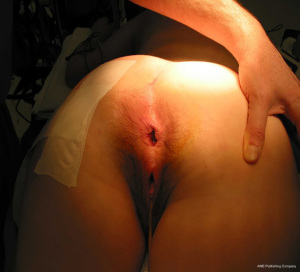
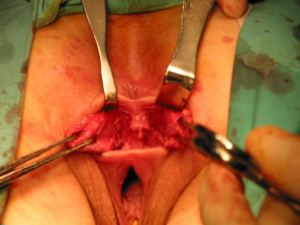
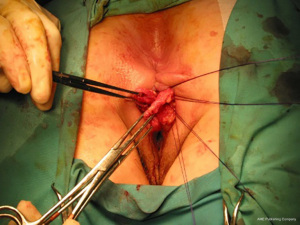
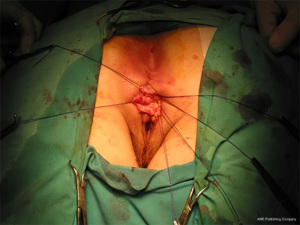
The anatomic goal of an overlapping sphincter repair procedure is correction of an anterior sphincter defect. Retracted ends of the sphincter are delineated and dissected (Figures 2,3), these may be scarred and involve external or both external and internal sphincter. Care should be taken to avoid extensive dissection of the muscle from the ischiorectal fossa to avoids neurological damage that can lead to further muscle atrophy and failure. When doing a repair, trying to separate and individually repair the internal and external sphincter is rarely feasible. Repair en mass of the external and internal sphincter together in doing the overlap has been the standard. The two ends are then overlapped and sutured together without tension to prevent ischemic necrosis (Figures 4,5). If the ends cannot be overlapped they can be repaired in end to end fashion and even augmented with biologic mesh (7). Redo repairs present an added challenge due to distorted anatomy. Endosonographic evaluation for the muscle quality may guide management as thin muscle and poor tissue quality, particularly in a redo situation, could result in another failed repair.
Use of biofeedback, SNM, and/or conservative nonsurgical care may be required to optimize function after a sphincter repair. Factors that have been shown to be predictive of a poor outcome include: low preoperative fecal incontinence score, obesity, residual sphincter defect post repair, pudendal neuropathy, associated pelvic floor injury, age as it is associated with decreased anal canal pressure, decreased rectal compliance, increased fibrosis of the internal anal sphincter, poor tissue quality, as well as greater than two prior repairs. These factors should be used to guide patient selection, preoperative counseling, and management of postoperative expectations.
In conclusion, sphincter repair may be offered to a young patient with associated incontinence or RVF after obstetrical injury. Sphincteroplasty is used as a primary method of repair for selected RVF associated with a sphincter defect. Long-term data demonstrates that there is a deterioration of continence over time. Sphincter repair as a primary treatment of incontinence improves function however post procedure continence is rarely perfect therefore it is important to evaluate severity of incontinence and set expectations for degree and durability of functional improvement. Care of patients with fecal incontinence should be viewed as a chronic disease and a combination of several treatments (both medical and surgical) may be required to provide the best result for the patient. Adjustments in the treatment approach may be required throughout the patient’s lifetime.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lucia Oliveira, Steven D. Wexner and Sarah A. Vogler) for the series “The Pelvic Floor and Anorectal Disorders” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-21-41/coif). The series “The Pelvic Floor and Anorectal Disorders” was commissioned by the editorial office without any funding or sponsorship. TLH is the president elect of the Society of Pelvic Surgeons. This society deals with issues of the pelvis. There is no real conflict in this paper. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- PMA P080025: FDA Summary of Safety and Effectiveness Data, Medtronic InterStim@ Therapy System, March 14, 2011.
- Oliveira L, Pfeifer J, Wexner SD. Physiological and clinical outcome of anterior sphincteroplasty. Br J Surg 1996;83:502-5. [Crossref] [PubMed]
- Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum 2012;55:482-90. [Crossref] [PubMed]
- Slade MS, Goldberg SM, Schottler JL, et al. Sphincteroplasty for acquired anal incontinence. Dis Colon Rectum 1977;20:33-5. [Crossref] [PubMed]
- Zutshi M, Tracey TH, Bast J, et al. Ten-year outcome after anal sphincter repair for fecal incontinence. Dis Colon Rectum 2009;52:1089-94. [Crossref] [PubMed]
- Barbosa M, Glavind-Kristensen M, Moller Soerensen M, et al. Secondary sphincter repair for anal incontinence following obstetric sphincter injury: functional outcome and quality of life at 18 years of follow-up. Colorectal Dis 2020;22:71-9. [Crossref] [PubMed]
- Zutshi M, Ferreira P, Hull T, et al. Biological implants in sphincter augmentation offer a good short-term outcome after a sphincter repair. Colorectal Dis 2012;14:866-71. [Crossref] [PubMed]
Cite this article as: Spivak AR, Hull TL. The role of sphincter repair for fecal incontinence. Ann Laparosc Endosc Surg 2022;7:11.


