
Best conservative options for fecal incontinence
Best conservative options for fecal incontinence (FI)
FI is defined as the uncontrolled passage of feces or gas, and is associated with diverse and often multifactorial causes including underlying functional, iatrogenic, and anatomic pathologies (1,2). It is estimated that nearly 20% of women over the age of 45 experience an episode of FI at least once per year, and patients prefer the term “Accidental Bowel Leakage” rather than FI when discussing these concerns with their physician (3). Regardless of the etiology of a patient’s FI, therapy is often initiated with conservative treatments that allow patients to avoid invasive surgical procedures (2). In the following chapter, we discuss the various methods utilized to conservatively treat FI. When these measures fail, patients may be candidates for surgical management including sacral neuromodulation (1,2,4-6),or anal sphincteroplasty (2) and these techniques will be discussed elsewhere in this series.
Dietary modification, fiber supplementation, and pharmacologic adjuncts
Diet modification is among the most commonly used therapies as it is intuitive for patients and often trialed prior to seeking formal medical attention. One study examined the dietary habits of 188 individuals with FI and demonstrated that about two-thirds of participants utilized diet modification techniques in the management of their FI. These strategies were near even in their distribution between those who used either restrictive (avoidance or consuming less of a food that worsens FI symptoms) or adoptive (consuming more food perceived to alleviate FI symptoms) practices. Certain foods seem to be more common to restrictive therapy, including caffeinated beverages, artificial sweeteners, fatty/greasy foods, fruits (including dried fruits and fruit juices), and spicy food. The most common adaptive therapy foods being those higher in fiber and in some cases those with probiotics. Despite following these general trends, however, the choice of which foods to restrict and which to adopt is often determined on a personalized basis in consultation with a dietitian (7).
The use of supplemental fiber has also been trialed as a conservative treatment for FI secondary to variation in stool consistency. In a study by Bliss et al., 39 individuals suffering from FI were divided into three groups with no significant difference in proportion of incontinence episodes at baseline (all >50% proportion incontinent stools). The groups were then assigned to either psyllium supplement, gum arabic supplement, or a pectin placebo over a 31-day study period. Results ultimately demonstrated a significant reduction in the proportion of incontinent stools in both the psyllium and gum arabic supplement groups to less than half that of the group ingesting the placebo (17–18% incontinent stools with fiber supplementation, 50% incontinent stools with placebo) (8).Bliss et al. further worked to characterize the tolerance of dietary fiber supplementation in regard to symptoms of GI upset including nausea, belching, feeling of fullness, abdominal cramping, and flatus. The study used daily symptom evaluations of 189 individuals at baseline and throughout several steady dose adjustments of either placebo, psyllium, gum arabic, or carboxymethylcellulose (CMC) over a 52-day trial period encompassing baseline, dose adjustment, steady dose segment 1, steady dose segment 2 periods. Ultimately there was no significant difference demonstrated in any of the measured GI symptoms either between the various supplement groups or between the steady dose adjustment groups compared to baseline symptom scores (9).
Various medical therapies have also demonstrated efficacy in the management of FI. Absorbent medications, such as bismuth salicylate, can be used in the setting of increased intestinal fluid secretion leading to diarrhea and worsening of FI. Cholestyramine works as a bile salt binding agent, as bile salts within the colon act as cathartic agents pulling in excess water and electrolytes. This is specifically useful in patients who have previously undergone ileocolic resection or cholecystectomy and have subsequently reduced bile salt absorption capacity (10). Anti-motility agents such as loperamide and diphenoxylate (commercially available in diphenoxylate-atropine) have efficacy in slowing intestinal motility and increasing transit time by binding to the intestinal µ receptor. Other opioid agents such as codeine work via the same mechanism but come with increased risk of CNS effects and dependence. A Cochrane review from 2013 examined 16 trials with a total of 558 participants examining the use of medical therapy to treat FI. They demonstrated limited consistent efficacy of anti-diarrheal agents in the treatment of FI given diverse patient populations and etiologies of FI across the examined trials. Data regarding the efficacy of these agents compared to other existing therapies remains lacking (11). Though there is some literature to support all of the outlined treatment modalities, the most practical approach is the use of combined supplemental fiber and dietary modification to avoid diarrhea, and sequentially add anti motility agents as indicated. It is assumed that simple causes of FI such as diarrhea will be controlled prior to moving on to surgical management.
Transanal irrigation/bowel training
Transanal irrigation encompasses the use of enema therapy to intermittently empty the rectum and distal colon with the aim of avoiding episodes of FI. This therapy has been shown to have the highest utility in patients suffering from FI secondary to primary constipation and subsequent overflow as occurs in low anterior resection syndrome (12).
Minimal published data exists to support the use of transanal irrigation and validation studies have been plagued by difficulty in retaining study participants. That said, there is weak evidence with small population studies to suggest significant improvement in FI symptoms with the use of transanal irrigation (12,13). Based on the variable efficacy and compliance within the literature, efficacy seems to be tied to patient preference and subsequent tolerance and compliance with the therapy. Further studies with higher subject retention are needed to truly evaluate the efficacy of transanal irrigation.
Biofeedback therapy (BFT) and pelvic floor rehabilitation
BFT is defined as the use of instrument-guided objective measurement to gain awareness of physiologic function for the purposes of improving control over these functions. Instrumentation used for BFT for incontinence specifically includes cutaneous or intravaginal/intrarectal receptors, ultrasound (intrarectal/intravaginal, perineal), rectal balloons, digital guidance, and anorectal manometry (14).
BFT has been shown to demonstrate efficacy compared to no therapy in the control of FI, with a stable and statistically significant improvement in number of incontinent episodes throughout a 5-year study period (15). BFT is often used in combination with techniques of pelvic floor exercises/rehabilitationand electrical stimulation (16,17).
Pelvic Floor Therapy encompasses exercises of pelvic floor muscle training including pelvic floor muscle contractions of varying length and frequency (18).Pelvic floor exercises can be done either with or without biofeedback techniques (14).
A Cochrane Review in 2010 examined 21 studies and concluded that data was insufficient and available studies were methodologically inadequate to draw definitive conclusions regarding the efficacy of biofeedback. It did, however, assert that some data supported the use of biofeedback in combination with other therapies such as sacral nerve stimulation and sphincter/pelvic floor exercises as outlined above (19).
Bulking injections
Bulking injection is a form of treatment for FI that involves injection of various biocompatible bulking agents into the anal canal submucosal space above the dentate line. Common agents utilized include Silicon biomaterial (PTQ), carbon-coated microspheres (Durasphere), dextranomer in stabilized hyaluronic acid, which is also known as NASHA Dx (Figure 1) (20-22). Other agents previously trialed include autologous fat and Polyacrylate polyalcohol copolymers (23,24). It is important to note that among the currently utilized injectable modalities, only NASHA Dx have shown durable success.
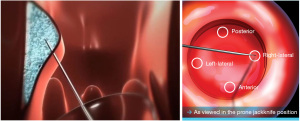
The singular agent approved for use in the United States is NASHA Dx. Efficacy of the agent was evaluated in a 2011 prospective randomized, double-blinded, and sham-controlled multicenter trial in Europe and the United States. The trial utilized a primary endpoint of >50% reduction in incontinence episodes, and results demonstrated 50% or greater reduction in incontinence episodes in 52% of the therapeutic treatment group participants as compared to 31% in the sham group at 6 months. Follow-up at 12 months indicated 50% or more reduction in FI episodes in 69% of patients in therapeutic group (sham group not measured at 12 months). Secondary endpoints included incontinence-free days, Fecal Incontinence Quality of Life (FIQL) score, and Cleveland Clinic Fecal Incontinence Score (CCFIS) following therapeutic injection compared to baseline. There was a significantly greater increase in mean number of incontinence-free days at 6 months in therapeutic group (3.1) as compared to the sham group (1.7). The mean number of incontinence-free days was significantly increased at the 12-month interval (7.9) from baseline (4.4) within the therapeutic treatment group. No significant difference was found in the change in CCFIS at 3 or 6 months between the two groups, though a significant decrease from baseline CCFIS was noted in the therapeutic treatment group alone at month 12 compared to baseline. The mean change in FIQL scores relative to baseline were significantly greater in the active treatment group for FIQL score categories of coping and behavior, but not for lifestyle, depression/self-perception, and embarrassment. At 12 months, there was a significant increase in FIQL score compared to baseline within the therapeutic treatment group for all 4 FIQL categories (22).
A Cochrane Review from 2013 examined the efficacy of all injectable bulking agents. It concluded that only the NASHA Dx injectable demonstrated significant improvement in continence, as outlined above. Though studies have been published in support of other injectable bulking agents, there is insufficient evidence to support their efficacy given the low quality of their studies, the lack of long-term outcome tracking, and lack of patient satisfaction reporting to accompany objective continence score analysis (25).
Subsequent data from the same NASH Dx study group published by Mellgren et al. in 2013 examined sustained response in the therapeutic treatment group at 36 months post-treatment using the same end-points as outlined above. The primary endpoint of 50% or greater reduction in incontinence episodes compared to baseline in the therapeutic group had a sustained significant response at 36 months (52% at 36-month, 52% at 6-month interval, as above). The significant increase in FIQL score as well as significant decrease in CCFIS were also sustained at the 36-month follow-up as compared to baseline (25,26). It is also important to note that most study subjects in the injectable trials required 2 injections to achieve efficacy.
Currently, there is an ongoing clinical trial of long-term efficacy of NASHA Dx of 277 patients with 36-month outcomes (NCT01647906) and the results of this trial could help change the recommendations on injectable therapy. Currently, the available data has shown that most patients need a second injection to obtain efficacy, and there is a high rate of positive response in the placebo group in these trials. A 2015 study from Techniques in Coloproctology indicated that some of the patients demonstrated migration of the injected agent over time and incontinence scores increased as the injected agent wore away over time (26,27). Long term data is needed to update these recommendations.
Posterior tibial nerve stimulation
The technique of percutaneous tibial nerve stimulation (PTNS), involving either a transcutaneous or a percutaneous approach, was first described in 1983 by McGuire et al. as a novel technique in the treatment of urologic disorders including urinary urge incontinence and overactive bladder (28). The technique was then described as a therapeutic strategy for FI by Shafik et al. in 2003 specifically in patients with FI secondary to uninhibited rectal contraction or uninhibited anal sphincter relaxation (29).
The techniques involved in this therapy differ in their approach to stimulation of the tibial nerve, either transcutaneous or percutaneous. Transcutaneous stimulation is completed by the method first described by Queralto et al. in 2006 and involves use of a transcutaneous electrical nerve stimulation (TENS) unit with placement of a negative electrode posterior to the medial malleolus and a positive electrode 10cm proximal to the electrode. Correct placement of the electrode is confirmed by observing toe contraction with electrode activation. Adequate stimulation is determined by intensity just under that of motor stimulation. This was originally described as 20-minute increments daily for a period of 4 weeks (30). In a 2013 randomized study by George et al., transcutaneous TNS and percutaneous TNS were compared to a placebo group of sham transcutaneous TNS with primary endpoint of >50% reduction in episodes of FI. Results demonstrated that 9/11 patients in the percutaneous arm, 5/11 in the transcutaneous arm, and 1/8 met the primary endpoint successfully (P=0.035, Fisher’s Exact Test). All patients with response were reportedly fully continent at end of the 6-week study period. Secondary outcomes included St. Mark’s Continence Score, Rockwood FI-specific Quality of Life score, and SF-36 Quality of Life score showed improvement but no significant difference between therapeutic and control arms of the study (31).
A placebo controlled randomized trial of tibial nerve stimulation was reported in 2015, known as the CONFIDeNT trial. This trial randomized 227 patients to PTNS (115 patients) vs. Sham (112 patients.) A 50% reduction in FI episodes was noted in 38% of the treatment group vs. 31% of the Sham group, indicating no major clinical benefit (32).
A 2019 meta-analysis by Sarveazad et al. sought to examine the efficacy of PTNS in reducing FI episodes. The studies included utilized a mixture of patients who had undergone PTNS (249 total individuals, mixed between those who had undergone percutaneous and transcutaneous), compared to control patients (239 total). The analysis found a significant decrease in FI episodes with the use of PTNS in patients with FI compared to control, without heterogeneity between the 4 studied included studies. Interestingly, the meta-analysis also examined available data in how PTNS affects various physiologic function involved in FI control. It found no significant impact on incontinence score, resting sphincter pressure, squeezing sphincter pressure, or maximum tolerable anal sphincter pressure. This data therefore does not provide an answer to the question of mechanism by which PTNS functions to alleviate FI episodes, though supports its overall efficacy (33).
Medical mechanical inserts
Anal insert
According to a systematic review that included 6 cross-over and prospective pilot trials, anal mechanical inserts are effective and satisfactory for the conservative treatment of FI. It is to be noted, however, that limited pooling of data regarding safety and efficacy across all modern anal insert devices limits overall evaluation of their clinical utility. The primary drawback to use of these devices continues to be device intolerance from patient discomfort (34).
The Renew Medical insert (Renew Medical Inc., Foster City, CA, USA) is a mechanical anal insert developed for the treatment of FI (Figure 2) (35). The device is marketed as a continuous use, silicon anal insert device used to prevent solid and liquid stool leakage associated with FI. Each device is single-use and expels naturally with a bowel movement. The Renew Medical insert has been validated specifically by a single arm, non-randomized study that examined its efficacy using primary end points of 50% or greater reduction in FI episodes, as well as secondary endpoint/metric of subjective improvement consisting of a hybrid Fecal Incontinence Score consisting of CCFIS. Among the 83 patients who completed the full 12-week trial period, there was a 77% rate of achieving the primary outcome with >50% reduction in FI episodes. Additionally, FI scores were improved by 32.4% throughout the course of the 12-week study period (36).

Vaginal insert
The Eclipse Vaginal insert (Pelvalon, Inc.) (Figure 3) is an inflatable vaginal insert that functions by way of temporary rectal occlusion with user-controlled inflation of a posterior-directed intra-vaginal balloon. The device is produced in several sizes and initiation of therapy using the Eclipse system requires fitting by a physician or nurse who is trained in the fitting process. A Trial Insert is utilized for the first 2 weeks to gauge clinical utility and patient comfort with device size. Permanent sizing can then be determined by this trial period. It can be safely utilized in patients with prior hysterectomy (37,38).
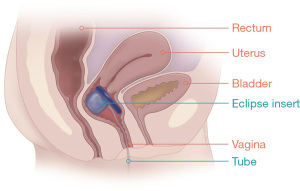
The Eclipse Vaginal insert has been clinically evaluated in the 2015 LIFE study, which involved a total of 61 participants in an intention-to-treat group (110 originally entered study but failed fitting stage) with primary end-point of 50% or greater reduction in FI episodes. Following study period of 1 month, 78.7% of intention-to-treat participants achieved clinical success, with 41.1% achieving complete continence. Secondary endpoints included incontinence episodes in a 2-week period, FIQL score, and Modified Manchester Health Questionnaire subscales. Mean incontinence episodes in the treatment group demonstrated significant decrease from baseline 11.6 to 2.1. Additionally, there was significant improvement in both overall score and subscale scores from both FIQL as well as the Modified Manchester HQ within the treatment group. Additionally, of the 56 intention-to-treat participants, 54 (96%) reported that they could not feel the insert (48%) or that they could feel it but were comfortable (48%) (37). The long-term efficacy of the product has since been evaluated at the 12-month end point in the same population by the LIBERATE clinical trial (NCT02428595), with primary endpoint being 50% or greater reduction in FI episodes at 3, 6, and 12 months of insert use. Of the 73 intention-to-treat participants analyzed at the 3-month interval, 73% achieved primary outcome of >50% reduction in FI episodes. This effect was consistent at 6-month interval with 71% and at 12-month interval with 70% achieving the primary outcome. Complete continence was achieved in 32%, 38%, and 34% at 3-, 6-, and 12-month intervals of the intention-to-treat group, respectively. Secondary outcomes included significant decrease in St. Mark’s score and significant increase in FIQL score at the 12-month interval (39).
All of the conservative treatments in this chapter can be considered as part of a comprehensive treatment strategy for FI, with patients who fail to improve then moving on to more advanced therapy such as sacral neuromodulation or sphincteroplasty if appropriate.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lucia Oliveira, Steven D. Wexner and Sarah A. Vogler) for the series “The Pelvic Floor and Anorectal Disorders” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-2022-04/coif). The series “The Pelvic Floor and Anorectal Disorders” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ivatury SJ, Wilson LR, Paquette IM. Surgical Treatment Alternatives to Sacral Neuromodulation for Fecal Incontinence: Injectables, Sphincter Repair, and Colostomy. Clin Colon Rectal Surg 2021;34:40-8. [Crossref] [PubMed]
- Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons' Clinical Practice Guideline for the Treatment of Fecal Incontinence. Dis Colon Rectum 2015;58:623-36. [Crossref] [PubMed]
- Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women's health study: prevalence and predictors. Int J Clin Pract 2012;66:1101-8. [Crossref] [PubMed]
- Hull T, Giese C, Wexner SD, et al. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum 2013;56:234-45. [Crossref] [PubMed]
- Johnson BL 3rd, Abodeely A, Ferguson MA, et al. Is sacral neuromodulation here to stay? Clinical outcomes of a new treatment for fecal incontinence. J Gastrointest Surg 2015;19:15-9; discussion 19-20. [Crossref] [PubMed]
- Goldman HB, Lloyd JC, Noblett KL, et al. International Continence Society best practice statement for use of sacral neuromodulation. Neurourol Urodyn 2018;37:1823-48. [Crossref] [PubMed]
- Croswell E, Bliss DZ, Savik K. Diet and eating pattern modifications used by community-living adults to manage their fecal incontinence. J Wound Ostomy Continence Nurs 2010;37:677-82. [Crossref] [PubMed]
- Bliss DZ, Jung HJ, Savik K, et al. Supplementation with dietary fiber improves fecal incontinence. Nurs Res 2001;50:203-13. [Crossref] [PubMed]
- Bliss DZ, Savik K, Jung HJ, et al. Symptoms associated with dietary fiber supplementation over time in individuals with fecal incontinence. Nurs Res 2011;60:S58-67. [Crossref] [PubMed]
- Halverson AL. Nonoperative management of fecal incontinence. Clin Colon Rectal Surg 2005;18:17-21. [Crossref] [PubMed]
- Omar MI, Alexander CE. Drug treatment for faecal incontinence in adults. Cochrane Database Syst Rev 2013;CD002116. [Crossref] [PubMed]
- Juul T, Christensen P. Prospective evaluation of transanal irrigation for fecal incontinence and constipation. Tech Coloproctol 2017;21:363-71. [Crossref] [PubMed]
- Martellucci J, Sturiale A, Bergamini C, et al. Role of transanal irrigation in the treatment of anterior resection syndrome. Tech Coloproctol 2018;22:519-27. [Crossref] [PubMed]
- Scott KM. Pelvic floor rehabilitation in the treatment of fecal incontinence. Clin Colon Rectal Surg 2014;27:99-105. [Crossref] [PubMed]
- Lacima G, Pera M, Amador A, et al. Long-term results of biofeedback treatment for faecal incontinence: a comparative study with untreated controls. Colorectal Dis 2010;12:742-9. [Crossref] [PubMed]
- Pager CK, Solomon MJ, Rex J, et al. Long-term outcomes of pelvic floor exercise and biofeedback treatment for patients with fecal incontinence. Dis Colon Rectum 2002;45:997-1003. [Crossref] [PubMed]
- Vonthein R, Heimerl T, Schwandner T, et al. Electrical stimulation and biofeedback for the treatment of fecal incontinence: a systematic review. Int J Colorectal Dis 2013;28:1567-77. [Crossref] [PubMed]
- Bartlett L, Sloots K, Nowak M, et al. Biofeedback for fecal incontinence: a randomized study comparing exercise regimens. Dis Colon Rectum 2011;54:846-56. [Crossref] [PubMed]
- Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev 2012;CD002111. [Crossref] [PubMed]
- Chan MK, Tjandra JJ. Injectable silicone biomaterial (PTQ) to treat fecal incontinence after hemorrhoidectomy. Dis Colon Rectum 2006;49:433-9. [Crossref] [PubMed]
- Altomare DF, La Torre F, Rinaldi M, et al. Carbon-coated microbeads anal injection in outpatient treatment of minor fecal incontinence. Dis Colon Rectum 2008;51:432-5. [Crossref] [PubMed]
- Graf W, Mellgren A, Matzel KE, et al. Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet 2011;377:997-1003. [Crossref] [PubMed]
- Shafik A. Perianal injection of autologous fat for treatment of sphincteric incontinence. Dis Colon Rectum 1995;38:583-7. [Crossref] [PubMed]
- Rosato G, Piccinini P, Oliveira L, et al. Initial results of a new bulking agent for fecal incontinence: a multicenter study. Dis Colon Rectum 2015;58:241-6. [Crossref] [PubMed]
- Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev 2013;CD007959. [Crossref] [PubMed]
- Mellgren A, Matzel KE, Pollack J, et al. Long-term efficacy of NASHA Dx injection therapy for treatment of fecal incontinence. Neurogastroenterol Motil 2014;26:1087-94. [Crossref] [PubMed]
- Guerra F, La Torre M, Giuliani G, et al. Long-term evaluation of bulking agents for the treatment of fecal incontinence: clinical outcomes and ultrasound evidence. Tech Coloproctol 2015;19:23-7. [Crossref] [PubMed]
- McGuire EJ, Zhang SC, Horwinski ER, et al. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol 1983;129:78-9. [Crossref] [PubMed]
- Shafik A, Ahmed I, El-Sibai O, et al. Percutaneous peripheral neuromodulation in the treatment of fecal incontinence. Eur Surg Res 2003;35:103-7. [Crossref] [PubMed]
- Queralto M, Portier G, Cabarrot PH, et al. Preliminary results of peripheral transcutaneous neuromodulation in the treatment of idiopathic fecal incontinence. Int J Colorectal Dis 2006;21:670-2. [Crossref] [PubMed]
- George AT, Kalmar K, Sala S, et al. Randomized controlled trial of percutaneous versus transcutaneous posterior tibial nerve stimulation in faecal incontinence. Br J Surg 2013;100:330-8. [Crossref] [PubMed]
- Knowles CH, Horrocks EJ, Bremner SA, et al. Percutaneous tibial nerve stimulation versus sham electrical stimulation for the treatment of faecal incontinence in adults (CONFIDeNT): a double-blind, multicentre, pragmatic, parallel-group, randomised controlled trial. Lancet 2015;386:1640-8. [Crossref] [PubMed]
- Sarveazad A, Babahajian A, Amini N, et al. Posterior Tibial Nerve Stimulation in Fecal Incontinence: A Systematic Review and Meta-Analysis. Basic Clin Neurosci 2019;10:419-31. [Crossref] [PubMed]
- Buono K, Davé-Heliker B. Mechanical inserts for the treatment of faecal incontinence: A systematic review. Arab J Urol 2019;17:69-76. [Crossref] [PubMed]
- Renew Medical. Available online: https://renew-medical.co.uk/products/renew-insert; Accessed 9/27/21.
- Lukacz ES, Segall MM, Wexner SD. Evaluation of an Anal Insert Device for the Conservative Management of Fecal Incontinence. Dis Colon Rectum 2015;58:892-8. [Crossref] [PubMed]
- Matthews CA, Varma MG, Takase-Sanchez MM, et al. Characteristics Associated With Successful Fitting of a Vaginal Bowel Control System for Fecal Incontinence. Female Pelvic Med Reconstr Surg 2016;22:359-63. [Crossref] [PubMed]
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol 2015;125:540-7. [Crossref] [PubMed]
- Varma MG, Matthews CA, Muir T, et al. Impact of a Novel Vaginal Bowel Control System on Bowel Function. Dis Colon Rectum 2016;59:127-31. [Crossref] [PubMed]
Cite this article as: Price AD, Paquette IM. Best conservative options for fecal incontinence. Ann Laparosc Endosc Surg 2022;7:17.


