
Pelvic floor anatomy
Introduction
The pelvic anatomy is somewhat challenging to both surgeons and anatomists. It is a narrow and deep region that encompasses intestinal, gynecologic, and urologic viscera, vessels, nerves, and fascial attachments. Understanding the pelvic anatomy in detail is difficult in vivo. Knowledge obtained from anatomic dissections and descriptions must be associated with data from physiologic and endoscopic investigations (1).
The dome-shaped pelvic floor is composed of striated muscle sheets, nerves, ligaments, and fascia and traversed by the urethra, vagina, and anal canal. The pelvic floor not only supports the pelvic viscera, but also has a key role in promoting urinary and anal continence. Thus, the pelvic floor is a multifunctional structure. These functions are largely attributed to its fibroelastic network, interconnecting organs, and virtual spaces to the osseous pelvis (2-4).
Rather than simply traversing the levator hiatus, pelvic viscera have an important role in the pelvic floor structure, configuration, and function. The anatomical and functional integration is due to sphincter mechanisms as well as other muscles and their attachments. Thus, because of their integrated anatomical and physiological aspects, viscera are, frankly, considered as part of the pelvic floor (4).
Osseous pelvis
The osseous pelvis supports the pelvic viscera, floor and its attachments, and transfers the weight of the torso to the lower legs. It includes the hip (pelvic or innominate) bones formed by the ilium, ischium, and pubis, as well as the sacrococcygeal segments. The pubic symphysis and sacroiliac joints are supported by ligaments, which allows movement and flexibility. Hormonal changes during pregnancy further increase flexibility and the potential capacity of the pelvis. The sacral promontory (pectineal line of the pubis) and superior pubic ramus form the pelvic brim circumferentially, marking the pelvic inlet. The classic four broad pelvic types are gynecoid, anthropoid, android, and platypelloid, determined by the pelvic inlet shape, although the shape and size of the bony pelvis varies considerably.
The greater (“false”) pelvis supports the lower abdominal viscera above the pelvic brim, specifically the ileum and sigmoid colon. Below the pelvic brim, the lesser (“true”) pelvis is the most relevant to the pelvic organs and for support of the pelvic floor. The bilateral sacrotuberous and sacrospinous ligaments attach the ischial tuberosity and ischial spines to the sacrum within the lesser pelvis. Although substantial pelvic floor support relies on normal levator ani function, its innervation, and fascial attachments, the osseous pelvis and sacrotuberous and sacrospinous ligaments are crucial in maintaining pelvic stability (5).
Pelvic floor musculature
The pelvic muscles are divided into five categories including the pelvic floor muscles (levator ani), anal sphincter complex, pelvic sidewall muscles, and anterior perineal muscles (6-8).
Based on phylogenetics, the anal sphincter and pelvic floor muscles originate from two cloaca groups, the sphincteric group and lateral compressor group (9). The sphincteric group is present in almost all animals. In mammals, it is divided into two components - urogenital and anal (10). In primates, the anal component originates at the external anal sphincter. In reptiles and mammals, the pelvicaudal group is more differentiated and originates at the ischiococcygeus, pubococcygeus and ileococcygeus muscles. Most primates have an additional muscle fiber group, in close proximity to the inner border of the pelvicaudal muscle that attaches the rectum to the pubis. In humans, these fibers are more developed and form the puborectalis muscle.
Levator ani muscle
The levator ani muscle is also called the pelvic diaphragm and is the main pelvic floor component. The levator ani muscle is in a continuous state of contraction, similar to the external anal sphincter and some postural muscles, providing active support to the intrapelvic viscera against the intrabdominal pressure that helps to prevent pelvic organ prolapse (11). The levator ani consists of symmetric muscle sheets comprised of three striated muscles: ileococcygeus, pubococcygeus, and puborectalis. It may also include a variable fourth muscle, the coccygeous or ischiococcygeous (Figure 1). The ischiococcygeous muscle originates at the ischial spine and is then inserted into the lower sacral and upper coccygeal segments. The coccygeous muscle is contiguous with the sacrospinous ligaments and helps reinforce the posterior pelvic floor.
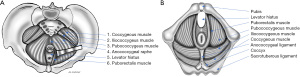
The ileococcygeus begins at the ischial spine and posterior portion of the obturator fascia (arcus tendineus levator ani), coursing medially and inferiorly to insert at the anoccygeal raphe and lateral aspects of the distal sacral and coccygeal bones, along with fibers from the opposite side. The pubococcygeus arises from the inner surface of the pubis and anterior aspect of the obturator fascia, runs dorsally along the anorectal junction to decussate with fibers of the opposite side at the anococcygeal raphe, and inserts on the anterior surface of the fourth sacral and first coccygeal segments. The pubococcygeous is also known as the pubovisceral because of its attachments to the pelvic viscera walls, including the pubovaginalis, puboperinealis, and puboanalis fibers. The latter refers to fibers attached to the anal canal at the intersphincteric plane and, with the puborectalis muscle, elevates the anus and anorectal junction.
The elliptic-shaped space between the two puborectalis muscles is the levator hiatus and the lower rectum, urethra, and either dorsal vein of the penis in males or vagina in females traverse it. Originating from the pelvic fascia, the hiatal ligament prevents the intrahiatal viscera from constricting during levator ani contraction. In addition, due to its crisscross fiber arrangement, the anococcygeal raphe may function as a dilator (12).
The puborectalis muscle is the most medial part of the levator ani muscle as a sturdy U-shaped loop of striated muscle that slings the anorectal junction to the posterior surface of the pubis (Figure 2).
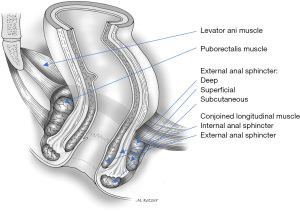
The puborectalis muscle is situated cephalad to the deep component of the external anal sphincter and the junction between the two muscles is relatively indistinct. As they both innervate from the pudendal nerves, the puborectalis has been considered as part of the external sphincter rather than the levator ani complex (12,13). Phylogenetic and anatomic studies have suggested that the puborectalis muscle may be part of the levator ani or the external anal sphincter (9,14). However, embryologically the puborectalis has a common primordium with the ileococcygeus and pubococcygeus muscles in that it is never connected to the external anal sphincter during the different developmental stages (15). Additionally, neurophysiologic studies have implied that innervation of these muscles may differ since stimulation of the sacral nerves results in electromyographic activity in the ipsilateral puborectalis muscle but not in the external anal sphincter (16). As a result of this controversy, the puborectalis has been considered as belonging to the levator ani and the external anal sphincter muscle groups (17).
Anal sphincter complex
The anal sphincter complex is formed by external anal sphincter, internal anal sphincter, and conjoined longitudinal muscles. The latter two muscles originate, respectively, from extensions of the circular and longitudinal layers of the rectum into the anal canal (18,19). The anal sphincter complex is supported anteriorly by the perineal body and its attachments to the anovaginal septum in females and the Denonvilliers’ fascia in males; posteriorly, it is attached to the coccyx by the anococcygeal ligament. Additional support is provided laterally by the levator ani and superficial transverse perineal muscles.
External anal sphincter
The external anal sphincter is a cylindrical-shaped striated muscle under voluntary control and is predominantly comprised of slow-twitch muscle fibers that are capable of prolonged contraction. Originally divided into subcutaneous, superficial, and deep parts, the external anal sphincter envelops the entire internal sphincter inner tube and caudally 1.0 cm beyond it (20). Goligher et al. (21) described it as a simple continuous sheet that, along with the puborectalis and levator ani muscles, forms a single funnel-shaped skeletal muscle (Figure 3). The external anal sphincter has also been described as subdivided into deep (deep sphincter and puborectalis) and superficial (subcutaneous and superficial sphincter) parts (22,23). Additionally, Shafik (12) proposed the three U-shaped loops system, but this schema was not clinically supported.
The external anal sphincter is most likely one single muscle unit that is attached to the coccyx by the anococcygeal ligament posteriorly and anteriorly to the perineal body, and not divided into layers as some authors have described. Nevertheless, differences in the arrangement of the external anal sphincter have been described between males and females (13). In the former, the upper half of the external anal sphincter is enveloped anteriorly by the conjoined longitudinal muscle, while the lower half is traversed by it. In the latter, the entire external anal sphincter is encased by a mixed fibers derived from both conjoined longitudinal and internal anal sphincter muscles.
On endosonographic evaluation, the puborectalis and external anal sphincters are seen as predominantly hyperechoic with a mean thickness ranging from 5 to 8 mm. Distinction is made by position, shape, and topography. Anal endosonography as well as magnetic resonance imaging (MRI) provide three-dimensional mapping of the anal sphincter. These imaging technologies show differences in the arrangement of the external anal sphincter between the sexes and can diagnose sphincter injuries (24-27). Decreased thickness of the external sphincter in older men has been demonstrated. In females, decreased thickness is also likely related to normal aging, although with coinciding external sphincter defects this may lead to incontinence. In addition, some degree of “anatomical asymmetry” of the external anal sphincter is expected, and this accounts for radial and longitudinal “functional asymmetry” that is observed during anal manometry (28).
The automatic continence mechanism is formed by the resting tone, maintained by the internal anal sphincter, and magnified by voluntary, reflex, and resting external anal sphincter activities. In response to conditions of threatened continence such as increased intra-abdominal pressure and rectal distension, the external anal sphincter and puborectalis muscles reflexively and voluntarily contract further to prevent fecal leakage. Normally, maximal voluntary contraction of the external anal sphincter can only be sustained for 30–60 seconds due to muscle fatigue, However, the external anal sphincter and pelvic floor muscles, unlike other skeletal muscles that are normally inactive at rest, maintain subconscious resting electrical tone through a reflex arc at the level of the cauda equina. Histology studies have shown that the external anal sphincter, puborectalis, and levator ani muscles have predominantly type I fibers, a peculiarity of skeletal muscles connecting tonic contractile activity (29). Nerve supply to the external sphincter is from the inferior rectal branch of the pudendal nerve (S-2, S-3) and the perineal branch of the fourth sacral nerve (S-4).
Internal anal sphincter
The internal anal sphincter, a smooth muscle, corresponds to the 2.5 to 4.0 cm distal expansion of the circular muscle layer of the distal rectum (Figure 4). The internal anal sphincter is in a constant state of maximal contraction due to myogenic and autonomic activities, providing a continuous barrier to the involuntary loss of rectal contents (18). On physical examination, the groove between the internal and external anal sphincters (intersphincteric sulcus) and lower rounded edge of the internal anal sphincter can easily be palpated at about 1.2 cm distal to the dentate line. Endosonographically, the internal anal sphincter is a uniform hypoechoic 2 to 3 mm thick circular band, but thickness increases with age (19).
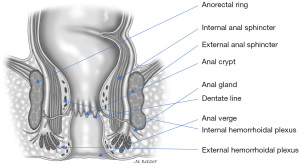
Conjoined longitudinal muscle
The conjoined longitudinal muscle is formed at the level of the anorectal ring, originating from the outer longitudinal rectal layer, and mixed with fibers of the puborectalis and deep portion of the external anal sphincter (30) (Figure 4). During its descent in the intersphincteric plane, this muscle may give rise to medial extensions that traverse the internal anal sphincter and contribute to the smooth submucosal muscle (musculus submucosae ani, Treitz muscle) (31,32) Ultimately, some of its fibers, known as the corrugator cutis ani muscle, traverse the lowermost portion of the external anal sphincter and insert into the perianal skin.
The conjoined longitudinal muscle works as a skeleton to support and bind together the internal and external sphincter complex and attach the anorectum to the pelvis (33). Some authors have considered that the meshwork formed by the conjoined longitudinal muscle functions as a support that prevents mucosal and rectal prolapse and can minimize functional deterioration of the anal sphincters after surgical division (34). Furthermore, the conjoined longitudinal muscle and its extensions to the intersphincteric plane and ischioanal fossa divide the adjacent tissues into subspaces and may have a role in the septation of thrombosed external hemorrhoids and containment of sepsis (31). Although controversial, Shafik (32) has ascribed the action of shortening and widening of the anal canal and eversion of the anal orifice to the conjoined longitudinal muscle and has proposed the term “evertor ani muscle”. In addition to this primary function during defecation, another limited role in maintaining anal continence has been proposed: a potentializing effect in maintaining an anal seal (32).
Anorectal ring and anorectal angle
The anorectal ring and anorectal angle are landmarks related to the puborectalis and the anorectal junction. The anorectal ring is a strong muscular ring around the anorectal junction that is a boundary of the anal canal and represents the upper end of the sphincter (the puborectalis) and is the upper border of the internal anal sphincter (20). The anorectal ring can be palpated on physical examination. Although there is no embryologic significance attributed to the anorectal ring, it is clinically significant as it is easily palpated on physical examination and an important landmark for the sphincteric barrier during surgical treatment of anorectal fistula or while determining the level of a low rectal anastomosis.
The anorectal angle is formed by the U-shaped sling of puborectalis muscle encircling the anorectal junction. Different theories have been postulated regarding the importance of the puborectalis and the anorectal angle in maintaining fecal continence. Parks et al. (35) noted that increasing intra-abdominal pressure pushes the anterior rectal wall down into the upper anal canal and occluding it by means of a flap valve mechanism, effectively creating a seal. However, it has been demonstrated that this flap valve mechanism, in fact, does not occur, but rather a continuous sphincteric occlusion-like activity attributed to the puborectalis occurs (36,37).
Pelvic sidewall muscles
The obturator internus and piriformis muscles line the pelvic posterolateral walls. The obturator internus originates from the pelvic surfaces of the ileum, ischium, and obturator membrane and exits the pelvis through the lesser sciatic foramen. It then inserts on the posterior surface of the greater trochanter. The parietal fascia envelopes the obturator internus and on its medial surface forms a thick anchoring structure to the levator ani muscle, the arcus tendineus levator ani.
The piriformis arises from the sacral anterolateral surface and exits the pelvis through the greater sciatic foramen. It also inserts on the superior border of the greater trochanter. Both muscles primarily rotate the thigh and, unlike the previous groups, lack any clinical relevance in anorectal disorders. However, they do act as liaisons between pelvic cavity and the extrapelvic spaces. For example, infections originating in the deep postanal space may propagate along the obturator internus and reach the ischional fossa.
Anterior perineal muscles and the perineal body
The perineal region is divided into two triangular shaped regions: posterior (or anal), relatively similar in both males and females, and anterior (or urogenital), comprising the urogenital diaphragm and external genitalia. The urogenital diaphragm is also known as the perineal membrane and is composed of the ischiocavernosus, bulbospongiosus, and superficial transverse muscles (Figure 5). Anteriorly-directed ischiocavernosus fibers arising from the posteromedial portion of the ischial tuberosities insert into the clitoris in females or de penile crus in males. The bulbospongiosus arises in the perineal body or central tendon in both males and females. In the latter, these muscles are separated in their course by the vaginal vestibule and insert into the dorsum of the clitoris. In males, the bulbospongiosus is fused at the midline by a raphe and covers the bulb and root of the corpus spongiosum of the penis. The superficial perineal muscle arises from the anterior aspect of the ischial tuberosity, transverses the perineum, and inserts into the perineal body.
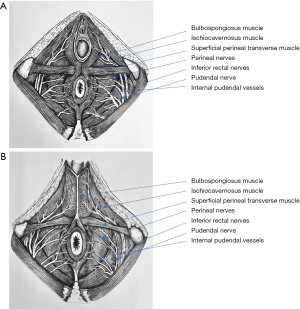
The perineal body is also known as the central tendon of the perineum and is a fibromuscular structure located between the vagina and anus. This structure is where both the anal and urogenital structures attach in the midsagital plane. Thus, the perineal body is formed by converging insertions of ligaments and muscles to the midline, including the urogenital diaphragm, rectogenital septum, and superficial transverse perineal, bulbocavernosus, external anal sphincter, and levator ani muscles. The perineal body is crucial to maintain pelvic support, particularly in females, and its rupture during delivery may predispose pelvic organ prolapse and incontinence.
Rectum
The rectum is a 12–15 cm segment of large bowel that follows the sacral concavity and terminates anteriorly at the coccyx. It then abruptly angulates back to cross the pelvic floor, at which point it forms the anal canal. Both the proximal and distal limits of the rectum have been debated (38). The rectosigmoid junction is regarded by pelvic surgeons as the sacral promontory and by anatomists as S3 level. Similarly, pelvic surgeons consider the distal limit of the rectosigmoid junction to be the anorectal ring while anatomists believe it to be the dentate line.
The upper third of the rectum is invested by peritoneum on its anterior and lateral aspects, while the middle rectum is only covered by peritoneum anteriorly. Since the anterior peritoneal reflection is at 9.0 to 7.0 cm from the anal verge in males, and 7.5–5.0 cm in females, the entire lower third of the rectum is extraperitoneal.
Similarly, the posterior aspect of the rectum is entirely extraperitoneal. Anatomically, the rectum lacks a mesorectum and the fatty tissue on its posterior aspect, including terminal branches of the inferior mesenteric artery and enclosed by the fascia propria, has been referred to by surgeons as the mesorectum. However, a more distinct mesorectum may be evident in patients with rectal prolapse. The mesorectum may be a targeted metastatic site from rectal cancer and can be removed without clinical sequelae (39).
Posteriorly, the rectum is related to the concavity of the sacrum and coccyx, median sacral vessels, and sacral nerve plexus roots. Anteriorly, it is related to the cervix uteri and posterior vaginal wall in females and behind the bladder, vas deferens, seminal vesicles, and prostate in males.
The rectum can be characterized by three lateral curves: the upper and lower curves are convex to the right and the middle curve is convex to the left. These curves correspond to the folds or valves of Houston on the intraluminal aspect. Typically, there are two folds on the left side at 7–8 cm and at 12–13 cm and one at 9–11 cm on the right side. The middle valve (Kohlrausch’s plica) is the most consistent and corresponds to the level of the anterior peritoneal reflection. However, these valves do not contain all the rectal wall layers and thus lack a specific function anatomically. However, clinically, the valves of Houston are very conducive for rectal biopsy as they are easily accessible with minimal risk of perforation (23).
Anal canal, anus, and anal verge
Although it is the terminal short segment of the intestinal tract, the anal canal has an ingenious and complex physiology and that accounts for its essential role in maintaining continence as well as its susceptibility to various diseases. The anus, or anal orifice, is an anteroposterior cutaneous slit that remains virtually closed at rest along with the anal canal due to the tonic circumferential contraction of the sphincters and presence of the anal cushions. The anal verge or margin (anocutaneous line of Hilton) delineates the lowermost border of the anal canal and is sometimes used as the level of reference for measurements taken during endoscopic examination. However, the dentate line is still considered as a more precise landmark. The difference between the anal verge and the dentate line is typically 1–2 cm. The epithelium distal to the anal verge acquires hair follicles, glands (including apocrine glands), and other features of normal skin, making it a potential site of perianal hidradenitis suppurativa.
The “anatomic” or “embryologic” anal canal is 2.0 cm long, extending from the anal verge to the dentate line, which corresponds to the proctodeal membrane (Figure 4).
The “surgical” or “functional” anal canal is longer, extending to approximately 4.0 cm (in males) from the anal verge to the anorectal ring (levator ani). The “long anal canal” concept was first introduced by Milligan and Morgan (20) and despite not being proximally marked by any apparent epithelial or developmental boundary, it has been considered useful as both a physiological and a surgical parameter. The anal canal is related to the coccyx posteriorly, while laterally it is related to the ischioanal fossa and inferior rectal vessels and nerves. Anteriorly, the perineal body and the distal part of the posterior vaginal wall are seen in females and the urethra in males.
The anorectal ring is at the level of the distal end of the ampullary part of the rectum and forms the anorectal angle and begins a region of higher intraluminal pressure. This definition correlates with digital, manometric, and sonographic examinations.
Fascial attachments of the rectum
The endopelvic fascia plays a major role in the support of pelvic organ and lines the pelvic walls and floor (parietal endopelvic fascia) and continues onto the internal organs as visceral pelvic fascia (Figure 6). The rectal fascia propria then becomes an extension of the pelvic fascia enclosing the rectum, fat, nerves, and blood and lymphatic vessels, primarily in the lateral and posterior extraperitoneal aspects of the rectum.
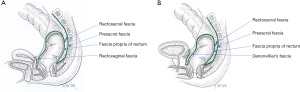
The lateral ligaments (lateral stalks) of the rectum, which are distal condensations of the rectal fascia propria, form a triangular-like structure with the base located on the lateral pelvic wall and the apex attached to the lateral aspect of the rectum. As noted by Church and colleagues (6), these ligaments have been the subject of “anatomical confusion and misconception”. They are essentially comprised of connective tissue and nerves. The middle rectal artery does not traverse the lateral stalks of the rectum; rather it sends minor branches uni- or bilaterally through the rectal stalks in approximately 25% of cases (40,41). Division of the lateral stalks using electrocautery is sufficient in the vast majority of cases. Ligation of the stalks implies retaining lateral mesorectal tissue that may preclude adequate lateral or mesorectal margins during rectal cancer surgery (39,42).
The presacral fascia, a thickened portion of the parietal endopelvic fascia, covers the concavity of the sacrum and coccyx, nerves, middle sacral artery, and presacral veins. Intraoperative rupture of the presacral fascia may cause severe hemorrhage due to injury of the underlying presacral veins in 4.6% to 7.0% of cases after rectal cancer surgery (43-45). These presacral veins are avalvular and communicate with the internal vertebral venous system through the basivertebral veins. They can achieve hydrostatic pressures of approximately 2–3 times the normal pressure of the inferior vena cava in the lithotomy position (43). Consequently, presacral hemorrhage can be life-threatening due to the high hydrostatic pressure. In addition, it can be difficult to control using conventional hemostatic measures due to retraction of the venous stump into the sacral foramen.
The rectosacral fascia is an anteroinferior directed thick fascial reflection from the presacral fascia to the fascia propria of the rectum at the S4 level. The rectosacral fascia has been erroneously referred to as Waldeyer’s Fascia. Although William Waldeyer did describe the entire pelvic fascia, he did not specifically emphasize the rectosacral fascia (6,46).
Anteriorly, the extraperitoneal rectum is separated from the prostate and seminal vesicles or vagina by a tough fascial investment known as the visceral pelvic fascia (or fascia of Denonvilliers) (47). Both the rectosacral and visceral pelvic fascia are important anatomical landmarks during rectal mobilization.
Pelvic ligaments and effects of pregnancy and delivery
Pelvic function has been compared to a “suspension bridge”, wherein the pelvic floor muscles contract against suspensory ligaments to support the organs from below and also actively participate during evacuation and continence (2,3,48). In fact, it has been demonstrated that, in conditions such as urinary stress incontinence, the ligaments rather than the muscles are the most vulnerable structures (3). In the female pelvis, these structures include the uterosacral and cardinal ligaments and attachment of paracolpium of vagina laterally (pubocervical fascia).
The puborectalis contracts against the pubic symphysis and forms the anorectal angle, but the ileococcygeous and the pubococcygeouscontract contract against the pelvic suspensory ligaments. Incontinence and pelvic organ prolapse are often attributed to older age and chronic straining during evacuation. However, during childbirth, overstretching and tearing of the pelvic floor muscles, nerves, and suspensory ligaments are often underestimated causes of incontinence and pelvic organ prolapse. Pregnancy may be one of the most important risk factors for pelvic floor dysfunction. Hormonal changes during pregnancy and their mechanical effects such as increased intra-abdominal pressure and changes in the axis of lumbar spine, result in alterations of the pelvic floor structure. These include downward pelvic floor displacement, decreased pelvic floor muscle contractions, and increased bladder and urethral mobility.
Increased laxity of the pelvis and ligaments during pregnancy occurs as a generalized effect of hormonal changes in the connective tissue. Pelvic floor damage may occur as early as during the first stage of labor, thus cesarean section in the active labor phase may not confer any protective effects in pelvic support or prevent pelvic floor dysfunction.
Vaginal delivery results in significant stretching of all the pelvic floor structures, especially in the first pregnancy, which is sometimes not completely reversible. Since the fetal head takes up most of the space in the birth canal, displacement of the pelvic organs and structures occurs during delivery. This is especially true during prolonged labor, potentially causing permanent structural and functional damage. During childbirth, pushing maneuvers rely mainly on abdominal pressure and uterine contractions. The pelvic floor does not have an active function, but instead relaxes enough to allow passage of the fetus.
The perineal body and anal sphincter are prone to intense stretching and significant pressure on their fibers. Vaginal delivery without visible perineal lacerations may still result in external anal sphincter damage. In up to 50% of cases, birth-related defects can be visualized on endoanal ultrasound (19). In addition, concomitant neurological injury can occur and may explain why surgical repair may not completely restore normal function. In fact, prolonged motor latencies in the internal anal sphincter may still persist for up to five months after vaginal delivery. Although pudendal neuropathy is less obvious than vaginal and perineal muscular lesions, it may still result in significant repercussions as it supplies the majority of anatomic structures that maintain pelvic support and continence. Consequently, neuropathic damage causes pelvic floor dysfunction such as anal and urinary incontinence, difficult evacuation, sexual dysfunction, and pelvic organ prolapse.
Vascular supply
The blood supply of the pelvic floor originates mostly from the internal pudendal artery, a branch from the internal iliac artery. The internal pudendal artery divides into lower rectal and perineal branches.
The superior rectal artery is a continuation of the inferior mesenteric artery; it descends into the sigmoid mesocolon to S3 level and then divides into right and left terminal branches to reach the posterior aspect of the rectum (49). Once they are within the rectal submucosa, these branches continue straight down to supply the lower rectum and anal canal, along with the middle and inferior rectal arteries.
Venous drainage follows its arterial supply. Blood from the rectum, through the inferior mesenteric vein, reaches the intrahepatic capillary bed through the portal vein. The internal and external pudendal veins connect respectively to the internal iliac and femoral veins. This collateral network, involving the middle rectal, internal iliac, and external iliac arteries could potentially prevent pelvic and lower extremity ischemia, in case of distal aortal occlusion (50,51).
The deep lymphatic drainage of the pelvic floor and rectum mainly flows into the internal iliac lymph nodes, following their respective vascular supply. Submucosal and subserosal rectal layers have an extensive network of lymphatic plexuses that drain into an extramural system of lymph channels (52). Lymphatic vessels from the perineum and genitals flow into the superficial inguinal lymph nodes.
Innervation
Pelvic floor muscles are innervated through a unique coordination of somatic, autonomic and central pathways.
The levator ani is innervated by its sacral roots on its pelvic surface (S2, S3, and S4 roots) and by the perineal branch of the pudendal nerve on its inferior surface. The puborectalis muscle receives additional innervation through the inferior rectal nerves. The external anal sphincter is primarily innervated from S2 roots, through the inferior rectal branches of the pudendal nerve (Figure 7).
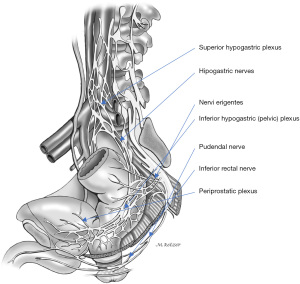
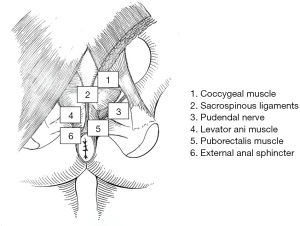
The pudendal nerve originates from the ventral branches of S2 to S4, courses between the piriformis and coccygeal muscles as it traverses through the greater sciatic foramen, arches around the ischial spine, and then enters back into the pelvis through the lesser sciatic foramen. The pudendal nerve then courses anteriorly to the sacrotuberous ligament, continuing along the lateral wall of the ischioanal fossa, through the Alcock’s canal or pudendal canal where it is enclosed by a sheath of the obturator fascia. The terminal branches of the pudendal nerve include the inferior rectal nerve, typically originating proximal to Alcock’s canal, the perineal nerve, and dorsal nerve of the penis or clitoris. The pudendal branches innervate the genitals, ischiocavernosus and bulbospongiosus muscles, perineum, anus, and urethral and external anal sphincters, and account for external genital sensation, urinary and anal continence, orgasm, and ejaculation.
The pelvic visceral responses are bilaterally represented on the primary motor cortex, located in the posterior portion of the frontal lobe (53). The primary motor cortex is connected to pelvic floor muscles nuclei in the sacral spinal cord. The motor neurons in the Onuf’s nucleus, located in the S2–S4 anterior horns of the spinal cord, supply the external anal and urethral sphincters and the pelvic floor muscles via pudendal nerve and muscular branches. Although the sphincter motor neurons within the Onuf’s nucleus are histologically and biochemically comparable to other somatic motor neurons, they seem to have some autonomic-like peculiarities. The sphincter motor neurons are smaller and more uniform in size as compared to other somatic motor neurons, and may show distinctive neuropharmacologic responses, such as a strong peptidergic activity (54-56). In addition, they can be relatively spared in some neurologic diseases, including amyotrophic lateral sclerosis and spinal muscular atrophy.
Skin innervation of the lower trunk, perineum, and proximal thigh is mediated through the iliohypogastric, ilioinguinal, and genitofemoral nerves (L1–L3). Sympathetic and parasympathetic innervation of the rectum closely follows its blood supply.
Conclusions
Although traditionally the pelvic floor has been divided into the anterior, middle, and posterior compartments, more recently it is thought to be a cohesive unit, a synergized composition of muscles, nerves, vessels, and ligaments, with a myriad of functions. As such, the interaction between the pelvic floor muscles and suspensory ligaments is crucial to maintaining normal pelvic function. Virtual reality models and innovative MRI, CT scan, and 3D ultrasound techniques have provided new insights to understand the intricate relationship among the pelvic structures.
Debilitating pelvic floor disorders such as pelvic organ prolapse and incontinence are usually related to injuries and deterioration of muscles, nerves, and ligaments that support and maintain normal pelvic function. Furthermore, stretching and tearing of these structures during childbirth are often underestimated causes of pelvic floor dysfunction. Understanding these anatomic concepts and the interaction of urological, gynecological, and anorectal compartments is essential in directing diagnosis and treatment of a myriad of pelvic floor disorders.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lucia Oliveira, Steven D. Wexner and Sarah A. Vogler) for the series “The Pelvic Floor and Anorectal Disorders” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-2022-06/coif). The series “The Pelvic Floor and Anorectal Disorders” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vesalius A. De humani corporis fabrica de recti intestini musculis. 1st ed. Brussels 1543:228.
- Petros PEP. The integral theory system. A simplified clinical approach with illustrative case histories. Pelviperineology 2010;29:37-51.
- Liedl B, Inoue H, Sekiguchi Y, et al. Update of integral theory and system for management of pelvic floor dysfunction in females. Eur Urol Suppl 2018;17:100-8. [Crossref]
- DeLancey JOL. Anterior pelvic floor in the female. In: Pemberton JH, Swash M, Henry MM, eds. The Pelvic Floor: its function and disorders. Elsevier Science Limited, 2002:13-28.
- Maldonado PA, Way CY. Pelvic organ prolapse. New concepts in pelvic floor anatomy. Obstet Gynecol Clin North Am 2016;43:15-26. [Crossref] [PubMed]
- Church JM, Raudkivi PJ, Hill GL. The surgical anatomy of the rectum – a review with particular relevance to the hazards of rectal mobilisation. Int J Colorectal Dis 1987;2:158-66. [Crossref] [PubMed]
- Kaiser AM, Ortega AE. Anorectal anatomy. Surg Clin North Am 2002;82:1125-38. v. [Crossref] [PubMed]
- Shafik A. A concept of the anatomy of the anal sphincter mechanism and the physiology of defecation. Dis Colon Rectum 1987;30:970-82. [Crossref] [PubMed]
- Wendell-Smith CP. Studies on the morphology of the pelvic floor [Ph.D. thesis]. London: University of London, 1967.
- Paramore RH. The Hunterian lectures on the evolution of the pelvic floor in non-mammalian vertebrates and pronograde mammals. Lancet 1910;1:1459-67. [Crossref]
- Parks AG, Porter NH, Melzak J. Experimental reflex of the reflex controlling mechanism of the pelvic floor muscles. Dis Colon Rectum 1962;5:407-14. [Crossref] [PubMed]
- Shafik A. A new concept of the anatomy of the anal sphincter mechanism and the physiology of defecation II. Anatomy of the levator ani muscle with special reference to puborectalis. Invest Urol 1975;13:175-82. [PubMed]
- Oh C, Kark AE. Anatomy of the external anal sphincter. Br J Surg 1972;59:717-23. [Crossref] [PubMed]
- Lawson JON. Pelvic anatomy II. Anal canal and associated sphincters. Ann R Coll Surg Engl 1974;54:288-300. [PubMed]
- Levi AC, Borghi F, Garavoglia M. Development of the anal canal muscles. Dis Colon Rectum 1991;34:262-6. [Crossref] [PubMed]
- Percy JP, Neill ME, Swash M, et al. Electrophysiological study of motor nerve supply of pelvic floor. Lancet 1981;1:16-7. [Crossref] [PubMed]
- Russell KP. Anatomy of the pelvic floor, rectum and anal canal. In: Smith LE. Practical guide to anorectal testing. New York: Ygaku-Shoin Medical Publishers, Inc., 1991:744-7.
- Jorge JMN, Wexner SD. Etiology and management of anal incontinence. Dis Colon Rectum 1993;36:77-97. [Crossref] [PubMed]
- Cuesta MA, Meijer S, Derksen EJ, et al. Anal sphincter imaging in fecal incontinence using endosonography. Dis Colon Rectum 1992;35:59-63. [Crossref] [PubMed]
- Milligan ETC, Morgan CN. Surgical anatomy of the anal canal: with special reference to anorectal fistulae. Lancet 1934;2:1150-6. [Crossref]
- Goligher JC, Leacock AG, Brossy JJ. The surgical anatomy of the anal canal. Br J Surg 1955;43:51-61. [Crossref] [PubMed]
- Garavoglia M, Borghi F, Levi AC. Arrangement of the anal striated musculature. Dis Colon Rectum 1993;36:10-5. [Crossref] [PubMed]
- Nivatvongs S, Gordon PH. Surgical anatomy. In: Gordon PH, Nivatvongs S, editors. Principle and practice of surgery for the colon, rectum and anus. St. Louis: Quality Medical Publishing, 1992:3-37.
- Bollard RC, Gardiner A, Lindow S, et al. Normal female anal sphincter: difficulties in interpretation explained. Dis Colon Rectum 2002;45:171-5. [Crossref] [PubMed]
- Fritsch H, Brenner E, Lienemann A, et al. Anal sphincter complex: reinterpreted morphology and its clinical relevance. Dis Colon Rectum 2002;45:188-94. [Crossref] [PubMed]
- Morren GL, Beets-Tan RG, van Engelshoven JM. Anatomy of the anal canal and perianal structures as defined by phased-array magnetic resonance imaging. Br J Surg 2001;88:1506-12. [Crossref] [PubMed]
- Williams AB, Bartram CI, Halligan S, et al. Endosonographic anatomy of the normal anal compared with endocoil magnetic resonance imaging. Dis Colon Rectum 2002;45:176-83. [Crossref] [PubMed]
- Jorge JM, Habr-Gama A. The value of sphincter asymmetry index in anal incontinence. Int J Colorectal Dis 2000;15:303-10. [Crossref] [PubMed]
- Swash M. Histopathology of pelvic floor muscles in pelvic floor disorders. In: Henry MM, Swash M, eds. Coloproctology and the pelvic floor. London: Butterworth-Heinemann, 1992:173-83.
- Fraser ID, Condon RE, Schulte WJ, et al. Longitudinal muscle of muscularis externa in human and nonhuman primate colon. Arch Surg 1981;116:61-3. [Crossref] [PubMed]
- Lunniss PJ, Phillips RK. Anatomy and function of the anal longitudinal muscle. Br J Surg 1992;79:882-4. [Crossref] [PubMed]
- Shafik A. A new concept of the anatomy of the anal sphincter mechanism and the physiology of defecation III. The longitudinal anal muscle: anatomy and role in sphincter mechanism. Invest Urol 1976;13:271-7. [PubMed]
- Courtney H. Anatomy of the pelvic diaphragm and anorectal musculature as related to sphincter preservation in anorectal surgery. Am J Surg 1950;79:155-73, illust. [Crossref] [PubMed]
- Haas PA, Fox TA Jr. The importance of the perianal connective tissue in the surgical anatomy and function of the anus. Dis Colon Rectum 1977;20:303-13. [Crossref] [PubMed]
- Parks AG, Porter NH, Hardcastle J. The syndrome of the descending perineum. Proc R Soc Med 1966;59:477-82. [Crossref] [PubMed]
- Bannister JJ, Gibbons C, Read NW. Preservation of faecal continence during rises in intra-abdominal pressure: is there a role for the flap valve? Gut 1987;28:1242-5. [Crossref] [PubMed]
- Bartolo DC, Roe AM, Locke-Edmunds JC, et al. Flap-valve theory of anorectal continence. Br J Surg 1986;73:1012-4. [Crossref] [PubMed]
- Jorge JMN, Bustamante-Lopez LA, Froehner I Jr. Anatomy of the anorectal region and pelvic floor. In: Oliveira LCC (ed). Anorectal Physiology. Springer Nature Switzerland, 2020.
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Boxall TA, Smart PJG, Griffiths JD. The blood supply of the distal segment of the rectum in anterior resection. Brit J Surg 1963;399-404. [PubMed]
- Wilson PM. Anchoring mechanisms of the anorectal region. S Afr Med J 1967;141:1138-43.
- Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986;2:996-9. [Crossref] [PubMed]
- Wang QY, Shi WJ, Zhao YR, et al. New concepts in severe presacral hemorrhage during proctectomy. Arch Surg 1985;120:1013-20. [Crossref] [PubMed]
- Zama N, Fazio VW, Jagelman DG, et al. Efficacy of pelvic packing in maintaining hemostasis after rectal excision for cancer. Dis Colon Rectum 1988;31:923-8. [Crossref] [PubMed]
- Jorge JMN, Habr-Gama A, Souza AS Jr, et al. Rectal Surgery Complicated by massive presacral hemorrhage. Arq Bras Cir Dig 1990;5:92-5.
- Crapp AR, Cuthbertson AM. William Waldeyer and the rectosacral fascia. Surg Gynecol Obstet 1974;138:252-6. [PubMed]
- Tobin CE, Benjamin JA. Anatomical and surgical restudy of Denonvilliers' Fascia. Surg Gynecol Obstet 1945;80:373-88.
- Gold DM, Ende D. A review of the integral theory of pelvic organ prolapse and proposed concept of repair. Part 1 – Structural components and damage. Pelviperineology 2016;35:74-6.
- Lindsey I, Guy RJ, Warren BF, et al. Anatomy of Denonvilliers’ fascia and pelvic nerves, impotence, and implications for the colorectal surgeon. Br J Surg 2000;87:1288-99. [Crossref] [PubMed]
- Griffiths JD. Extramural and intramural blood supply of the colon. Br Med J 1961;1:323-6. [PubMed]
- Lindstrom BL. The value of the collateral circulation from the inferior mesenteric artery in obliteration of the lower abdominal aorta. Acta Chir Scand 1950;100:367-74. [PubMed]
- Block IR, Enquist IF. Studies pertaining to local spread of carcinoma of the rectum in females. Surg Gynecol Obstet 1961;112:41-6.
- Turnbull GK, Hamdy S, Aziz Q, et al. The cortical topography of human anorectal musculature. Gastroenterology 1999;117:32-9. [Crossref] [PubMed]
- Thor KB, de Groat WC. Neural control of the female urethral and anal rhabdosphincters and pelvic floor muscles. Am J Physiol Regul Integr Comp Physiol 2010;299:R416-38. [Crossref] [PubMed]
- Schellino R, Boido M, Vercelli A. The Dual Nature of Onuf's Nucleus: Neuroanatomical Features and Peculiarities, in Health and Disease. Front Neuroanat 2020;14:572013. [Crossref] [PubMed]
- Dobson HD, Pearl RK, Orsay CP, et al. Virtual reality: new method of teaching anorectal and pelvic floor anatomy. Dis Colon Rectum 2003;46:349-52. [Crossref] [PubMed]
Cite this article as: Jorge JMN, Bustamante-Lopez LA. Pelvic floor anatomy. Ann Laparosc Endosc Surg 2022;7:20.


