Laparoscopic right hemicolectomy: how I do it
Introduction
In 1991, laparoscopic colorectal surgery was introduced in the field of gastrointestinal surgery as the role of minimally invasive surgery continued to expand to treat abdominal conditions (1,2). Soon after, major centers such as the Mayo Clinic started reporting their early experience with large series of patients (3). Much debate ensued questioning the safety and adequacy of laparoscopic surgery for the treatment of cancer and concerns were raised around the globe by skeptics regarding the emerging minimally invasive approach. Such concerns were eventually laid to rest after major randomized clinical trials such as the COST trial demonstrated the favorable oncologic outcome for patients undergoing laparoscopic surgery (4-8). Recognizing the benefits of laparoscopic surgery, the early 21st century saw the gradual global implementation of minimally invasive surgery. In addition to the short-term benefits which included faster recovery and shorter length of stay compared to traditional open surgery, the long-term advantages of laparoscopic surgery included a lower rate of intestinal adhesions formation, fewer bowel obstructions, and a decrease risk for incisional ventral hernia. A large study from Kaiser Permanente in California looked at the outcome of 4,765 colorectal resections over a 3-year period and confirmed the positive long-term benefits of laparoscopy with a significant lower incidence of small bowel obstruction and incisional ventral hernia (9).
Since its initial description by Schlinkert at the Mayo Clinic in 1991, several approaches to laparoscopic right hemicolectomy have been reported (10-13). These various techniques depict a variety of methods for colon mobilization, control of the mesocolic vasculature, anastomosis construction, and specimen extraction sites. Previously the senior author of this publication has described the extracorporeal anastomotic technique for laparoscopic right hemicolectomy (10). In this technical paper, we would like to demonstrate the intracorporeal anastomosis approach for laparoscopic right hemicolectomy for cancer.
Surgical technique
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images/video. A copy of the written consent is available for review by the editorial office of this journal.
This case was a 58-year-old male who was diagnosed cecal adenocarcinoma. Right hemicolectomy with total mesocolic excision was done. Duration of the operation was 128 minutes. Blood loss was 150 milliliters. Length of hospital stay was 4 days.
Step by step
Step 1—patient preoperative preparation
In our practice, we currently administer the standard mechanical oral bowel preparation the day prior to the operation. The debate regarding bowel cleansing preoperatively has been ongoing for several decades and the recommendations have varied based on the scientific evidence.
Step 2—patient intraoperative preparation and positioning
The operation is performed under general endotracheal anesthesia. The patient is placed in the supine position with both upper extremities tucked to the side. The patient is secured across the shoulders’ prominence to the table with tape. A vertical footboard supports the soles of the feet for added security. An orogastric tube is inserted to decompress the stomach prior to establishing the pneumoperitoneum. The orogastric tube is removed at the completion of the operation. A urinary bladder catheter is introduced to monitor urine output during the case and it is usually removed within 12 hours of operation. Bilateral transversus abdominis plane (TAP) block is performed under ultrasound guidance prior to performing the operation. It is part of our multimodal postoperative pain management. Pneumatic compression stockings are applied. Intravenous antibiotics are administered prior to incision. Our preferred regimen is a third-generation cephalosporin along with metronidazole for patients without penicillin allergy. Deep venous thrombosis prophylaxis is given with subcutaneous unfractionated heparin. The abdomen is prepped and draped in a sterile fashion. The video monitor is placed at the right upper aspect of the surgical table (Figure 1).
Step 3—pneumoperitoneum insufflation and trocar placement
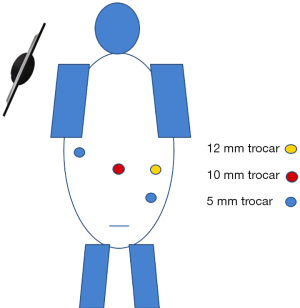
The operation is performed via a 4-trocar technique. The abdominal cavity is insufflated with a Veress needle through a 12 mm supraumbilical vertical incision. An additional 10 mm trocar is placed in the left mid lateral abdomen, a 5 mm trocar in the left lower quadrant, and a 5 mm trocar in the right mid abdomen laterally. The 12 mm trocar sites will be interchangeably used for a 10 mm camera and for the endoscopic stapler (Figure 2).
Step 4—mesocolic vessels division
Laparoscopic colorectal surgery requires frequent tilting of the operative table in various positions in order to provide exposure and repositioning the small bowel out of the way. Most of the operation is performed with the patient ride side rotated upward. Periodically the patient is titled in the Trendelenburg position or reverse Trendelenburg position depending on the phase of the operation. After exposing the right mesocolon, the ileocolic pedicle is identified and we proceed with the dissection from a medial to lateral approach. We use the Harmonic® Scalpel (Ethicon Endo-Surgery, Inc., OH, USA) for dissection. It is important to note that there is a variation in arterial anatomy for the right colic vasculature. In some patients, there is a common trunk for the ileocolic and right colic vessels while in other patients, they have separate takeoffs. Yet in a subgroup of patients, the right colic vessels are not present. A mesenteric window is opened around the base of the ileocolic pedicle which is reflected anteriorly away from the duodenum by creating a dissection plane behind the mesocolon and anterior to the retroperitoneum. After isolating the ileocolic vascular pedicle, the vessels are clipped with a Vas-o-clip® polymer locking ligation clips (Nanova Biomaterial, Inc., Columbia, USA) and divided with the energy source. If present separately the right colic pedicle is addressed in a similar fashion. For a standard right hemicolectomy, the middle colic vessels are preserved but if indicated by the clinical scenario, they can be divided similarly. After vascular division, blunt dissection is used to completely free the mesocolon from the retroperitoneum ensuring that the dissection remains anterior to the duodenum and head of the pancreas.
Step 5—hepatic flexure takedown
After vascular division, complete mobilization of the right colon is achieved by reflecting the colon from a lateral to medial and then top-down approaches. Using the energy device, the lateral attachments are taken down by incising initially and then dividing the colon attachments laterally. The greater omentum is divided coming along the mid transverse colon and separating it from the greater curvature of the stomach and eventually dividing the hepatic flexure attachments.
Step 6—dividing the bowel
The proximal bowel margin is marked at the terminal ileum followed by identification of the distal bowel margin at the transverse colon. The mesenteric and mesocolic aspects of the margins is further divided with the energy device as needed. An endoscopic linear stapler, Echelon FlexTM Endopath® 60 mm green cartilage (Ethicon Endo-Surgery, Inc.) is introduced through the 12 mm trocar and the bowel is transected, preferably with a single cartridge firing for each proximal and distal margins. The specimen is pushed towards lower abdomen out of the surgical field.
Step 7—intracorporeal anastomosis
A side-to-side intracorporeal anti-peristaltic anastomosis is performed. A single suture is initially placed to approximate the small bowel and colon. The suture can be anchored to the abdominal wall for adequate exposure and retraction. An enterotomy and colotomy are performed using the energy device and a single endoscopic stapler, Echelon FlexTM Endopath® 60 mm green cartilage (Ethicon Endo-Surgery, Inc.), is performed side to side to create the luminal aspect of the anastomosis. The open apex of the anastomosis is then closed with a running absorbable suture, V-LocTM (Medtronic, Minneapolis, USA). Additional single interrupted absorbable sutures can be used to reinforce some areas of the anastomosis. The mesenteric window can be left open or closed at the discretion of the surgeon. The trocars are removed under direct visualization and checked for bleeding. The fascia of the 10 and 12 mm sites are closed with an absorbable suture.
Step 8—specimen extraction
A 6 cm horizontal Pfannenstiel incision is made in the lower abdomen. An AlexisTM wound protector (Applied Medical, Rancho Santa Margarita, CA, USA) is placed and the specimen is exteriorized and sent for histologic evaluation. The fascia is closed using a running absorbable suture and the skin of the midline extraction site and trocar sites is closed with an absorbable subcuticular suture. An example of this technique is shown in Video 1 so you can follow these steps visually.
Comments
A minimally invasive approach to right colectomy provides several benefits to the patient and it is a rewarding operation for the surgeon. Complete mesocolic excision (CME) is preferred by our team in laparoscopic right hemicolectomy. CME provides more lymph node harvest and more accurate oncologic surgery and increases survival. CME is not inferior to standard laparoscopic surgery in terms of anastomotic leakage, blood loss, and overall postoperative complications. Traditional laparoscopic right hemicolectomy has a shorter operative time and lower conversion rate to open surgery (14). It is important to note that several techniques have been described and the variations encompass the different approaches to colon mobilization, control of the vascular pedicle, anastomosis construction, and specimen extraction site. These variations include pure laparoscopic technique, laparoscopic assisted, hand assisted, single port surgery, and more recently robotic surgery. The 2 main anastomotic techniques are intracorporeal and extracorporeal. All techniques are acceptable and it is important for the surgeon to be consistent in the technique he/she uses while at the same time being familiar with the other options which may prove useful under some circumstances. In the past years extracorporeal anastomosis was frequently performed in laparoscopic surgeries because the intracorporeal anastomosis technique required more experience. With the development of surgical experience and laparoscopic staplers, intracorporeal anastomosis is now more frequently performed. Studies have shown that although there is no significant difference in terms of anastomotic leakage and oncological results, the duration of hospitalization and even the operation time are shorter. Smaller incision is needed for the removal of the specimen, resulting in less wound complications and less pain (15). The general key principles are good visualization, good mobilization, proximal vascular pedicle control for adequate lymphadenectomy (which is critical for neoplastic condition), reliable and safe anastomotic construction technique, and extraction site wound protection to avoid wound infections or extraction site tumor recurrence in case of malignancy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Abe Fingerhut) for the series “The Expert’s Technical Corner: How I Do It” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-22-69/coif). The series “The Expert’s Technical Corner: How I Do It” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images/video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991;1:144-50. [PubMed]
- Schlinkert RT. Laparoscopic-assisted right hemicolectomy. Dis Colon Rectum 1991;34:1030-1. [Crossref] [PubMed]
- Dean PA, Beart RW Jr, Nelson H, et al. Laparoscopic-assisted segmental colectomy: early Mayo Clinic experience. Mayo Clin Proc 1994;69:834-40. [Crossref] [PubMed]
- Nelson H, Sargent DJ, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [Crossref] [PubMed]
- Chung CC, Ng DC, Tsang WW, et al. Hand-assisted laparoscopic versus open right colectomy: a randomized controlled trial. Ann Surg 2007;246:728-33. [Crossref] [PubMed]
- Kitano S, Inomata M, Mizusawa J, et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol 2017;2:261-8. [Crossref] [PubMed]
- Liu ZH, Wang N, Wang FQ, et al. Oncological outcomes of laparoscopic versus open surgery in pT4 colon cancers: A systematic review and meta-analysis. Int J Surg 2018;56:221-33. [Crossref] [PubMed]
- Deijen CL, Vasmel JE, de Lange-de Klerk ESM, et al. Ten-year outcomes of a randomised trial of laparoscopic versus open surgery for colon cancer. Surg Endosc 2017;31:2607-15. [Crossref] [PubMed]
- Klaristenfeld DD, McLemore EC, Li BH, et al. Significant reduction in the incidence of small bowel obstruction and ventral hernia after laparoscopic compared to open segmental colorectal resection. Langenbecks Arch Surg 2015;400:505-12. [Crossref] [PubMed]
- Wong JT, Abbas MA. Laparoscopic right hemicolectomy. Tech Coloproctol 2013;17:S3-9. [Crossref] [PubMed]
- Walmsley J, Max S, Hughes G, et al. A stepwise approach to laparoscopic right hemicolectomy with D2 excision. An illustrated video guide for training colorectal surgeons - a video vignette. Colorectal Dis 2021;23:2484-6. [Crossref] [PubMed]
- Fujita J, Uyama I, Sugioka A, et al. Laparoscopic right hemicolectomy with radical lymph node dissection using the no-touch isolation technique for advanced colon cancer. Surg Today 2001;31:93-6. [Crossref] [PubMed]
- Senagore AJ, Delaney CP, Brady KM, et al. Standardized approach to laparoscopic right colectomy: outcomes in 70 consecutive cases. J Am Coll Surg 2004;199:675-9. [Crossref] [PubMed]
- Anania G, Davies RJ, Bagolini F, et al. Right hemicolectomy with complete mesocolic excision is safe, leads to an increased lymph node yield and to increased survival: results of a systematic review and meta-analysis. Tech Coloproctol 2021;25:1099-113. [Crossref] [PubMed]
- Biondi A, Di Mauro G, Morici R, et al. Intracorporeal versus Extracorporeal Anastomosis for Laparoscopic Right Hemicolectomy: Short-Term Outcomes. J Clin Med 2021;10:5967. [Crossref] [PubMed]
Cite this article as: Oner M, Cipe G, Abbas MA. Laparoscopic right hemicolectomy: how I do it. Ann Laparosc Endosc Surg 2023;8:12.