
A detailed guide to endoscopic colonic stent insertion in obstructing colorectal cancer
Highlight box
Surgical highlights
• Endoscopic colonic SEMS insertion is a useful treatment option in the management of select patients with obstructing colorectal cancer that is performed in many colorectal and advanced endoscopic centres.
• We describe in detail the three major phases of this procedure—guidewire cannulation, fluoroscopy and SEMS deployment.
What is conventional and what is novel/modified?
• Many institutions utilise various methods and techniques for colonic SEMS insertion that will have both similarities and differences to our described method in terms of set-up, equipment and insertion technique.
What is the implication, and what should change now?
• This article provides a detailed step-by-step guide on how this procedure is performed at our institution, including pre-operative planning and equipment, procedural set-up as well as tips and troubleshooting suggestions for each step of the procedure.
Introduction
Colonic self-expandable metal stent (SEMS) insertion is a viable management option in the treatment of malignant large bowel obstruction (LBO) in select patients. The most common indication for colonic SEMS insertion is malignant LBO in patients with metastatic disease with palliative intent (1). It has also been considered as a ‘bridge to surgery’ option in patients with resectable disease who present with obstruction that would otherwise require an emergency resection and likely stoma formation, although the evidence remains conflicted (2). Stenting can also be performed for extra-colonic LBO, but with lower success rates (3). In cases of previous stent obstruction such as from tumour in-growth, SEMS can be re-deployed in a ‘stent-in-stent’ configuration (4).
Colonic SEMS should generally be avoided in patients with low rectal obstruction as this may cause significant pain and tenesmus. Caution should also be applied to patients receiving targeted anti-angiogenic therapy such as bevacizumab, due to a higher risk of stent perforation (1). Colonic SEMS can be inserted under direct vision endoscopically “through-the-scope (TTS)”, or less commonly “non-TTS” as a fluoroscopic radiological procedure (1).
We discuss the technical steps and the relevant preparation required for TTS insertion of a colonic SEMS with intra-operative images.
Preoperative preparations and requirements
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
All patients should have pre-operative computed tomography (CT) imaging with intravenous contrast to aid in procedural planning. It is important to identify the site of obstruction and potential stent placement, in particular to estimate an approximate length of tumour as this will influence the length of the stent that is deployed. A potential increased risk of perforation should be considered during SEMS deployment in the splenic and hepatic flexures due to the axial force exerted by the stent in these areas (5). This can be mitigated with tailored stent length selection, using longer lengths to relieve the pressure load on the colon.
Equipment
A long length 0.035 inch guidewire with a hydrophilic tip is required to cannulate the tumour lumen. We use a 450 cm length JagwireTM (Boston Scientific) for this purpose. A smaller calibre 0.025 inch guidewire is also commonly utilised in many centres worldwide, and is a viable alternative if available.
We utilise the WallFlexTM Colonic Stent (Boston Scientific), an uncovered nitinol SEMS which is commonly used in Australia. It is available in lengths of 60, 90 and 120 mm, and expands to 22–25 mm mid-body, and 27–30 mm at the end flanges.
Biliary access catheters, such as those used for ERCP [JagtomeTM RX Cannulating Sphincterotome or TandemTM XL Triple-lumen cannula (Boston Scientific)] should be available to facilitate contrast injection during fluoroscopy. CRETM Balloon Dilator (Boston Scientific) can also be used for this purpose depending on endoscopist preference.
Image intensifier should be available to provide intra-operative radiography and fluoroscopy. Patients should receive a fleet enema preoperatively. All staff within the operating room should wear individual lead protection.
Tips & pearls
- The SEMS length should allow at least 2 cm both proximal and distal coverage beyond the obstruction. In our experience, a 90 mm stent is the most used length, but tumours that traverse a bend or corner will require a longer 120 mm stent. Tumours in the upper rectum will require a shorter stent to limit symptoms of tenesmus caused by irritation of the stent on the anal side of the obstruction.
- It may be useful to have a medical equipment company representative present to troubleshoot any technical issues during the initial first few cases performed by the endoscopist.
Patient positioning
At our institution, the procedure is generally performed under general anaesthetic on the operating table. However, this is not mandatory as we recognise that the procedure is performed under conscious sedation in other centres. There are 2 main approaches to patient position partly based on endoscopist preference: left lateral decubitus or supine with frog leg.
Supine allows more space for the image intensifier C-arm with optimal interpretation of fluoroscopic images, and minimises patient repositioning should the SEMS insertion fail and an emergency operation is required. Left lateral decubitus positioning provides familiarity to the surgeon and can facilitate a successful stent insertion in an otherwise difficult situation with unprepared bowel. An extra table may be placed at the end of the operating table to rest various equipment during the procedure. Ideal room setup is demonstrated in Figure 1A.
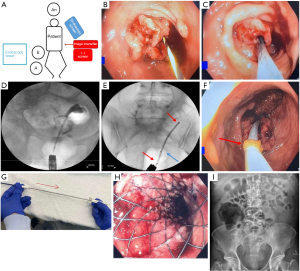
Step-by-step description
Guidewire cannulation
- Step 1: the colonoscope is inserted to the level of the obstruction, using minimal gas insufflation with carbon dioxide to avoid excess proximal colonic distension. Water insufflation can also be utilised as the bowel will be unprepared.
- Step 2: the guidewire can be pre-loaded into the catheter, and both inserted through the colonoscope. Once the tumour is encountered, the guidewire can be inserted through the obstruction under fluoroscopic guidance, with careful and sequential advancement of the catheter over the guidewire.
The guidewire is inserted through the scope with the assistant securing the distal end. Careful cannulation of the bowel lumen through the tumour is then performed with the guidewire (Figure 1B) once a potential lumen is identified. This can be difficult, especially with high grade obstruction or a friable tumour.
Regular radiography with the image intensifier should be performed to ensure progress of the wire and catheter into the colon proximal to the obstruction.
Tips & pearls
- During cannulation, multiple attempts may be required with constant repositioning of the colonoscope and guidewire into difference portions of the tumour, as the lumen is often not clearly visible. It may be helpful at this stage for the endoscopist to control the colonoscope with the assistant advancing and retracting the guidewire as needed.
Fluoroscopy
- Step 3: at this juncture, contrast is injected through a catheter that is railroaded over the guidewire through the colonoscope. Fluoroscopy allows for confirmation that the guidewire has passed the level of obstruction, exclusion of colonic perforation, and estimation of stricture length. This can be performed using a biliary access cannula or a CRETM Balloon Dilator, utilising its radiopaque markers as an aid in estimating stricture length.
Tips & pearls
- We recommend 50% diluted contrast as it is less viscous and therefore easier to administer. The use of a 10 mL syringe facilitates ease of injection.
- The balloon can be insufflated with air as an added visual aide to mark the proximal extent of the obstruction if required.
- Step 4: once fluoroscopy is performed, the catheter is pulled back under image intensifier guidance. During this process, the guidewire should be pushed forwards simultaneously to ensure it remains above the obstruction and should be grasped at the colonoscope working channel as soon as the catheter tip exits.
SEMS deployment
- Step 5: the deployment sheath, a flexible catheter containing the compressed SEMS, is then railroaded over the guidewire through the scope (Figure 1C).
It is important during this step that the endoscopist minimises migration of the colonoscope away from the tumour and maintains the lumen in view, while the assistant secures the guidewire taut to prevent both distal slippage out of the colon and proximal looping of the guidewire. The image intensifier should be used at regular intervals to confirm wire position during this manipulation process. Contrast pooling indicates the location of the proximal end of the obstruction (Figure 1D). There are radiopaque markers on the delivery system that aid accurate deployment, including markers that identify the proximal and distal ends of the stent. A third marker in-between defines the re-constrainment limit, after which the SEMS is not able to be re-sheathed (Figure 1E).
- Step 6: the sheath should be inserted until the distal flange of the SEMS is positioned distal to the obstruction. The distal flange can be visualised directly endoscopically by a yellow marker (Figure 1F). Once the sheath has been positioned across the obstruction, deployment of the SEMS can occur.
Tips & pearls
- A common pitfall is when the sheath has passed beyond endoscopic vision, but the distal end of the guidewire is still completely enclosed within the sheath and has not yet protruded out for the assistant to control. This occurs because the guidewire length is only slightly longer than the sheath length. To avoid this, it is important to ensure that excess amount of guidewire past the tumour is retracted. In addition, the sheath can be flushed with saline to allow the guidewire to ‘float’ through.
- Step 7: the assistant immobilises the proximal handle of the sheath while slowly sliding the distal handle proximally to retract the sheath constraining the SEMS (Figure 1G). The endoscopist must provide counter traction to prevent proximal migration of the stent during deployment and to position the distal end of the stent accurately. If the stent moves out of position during this process, the sheath can be re-advanced over the stent if the re-constrainment limit has not been exceeded.
Upon SEMS deployment, a flow of bowel contents from proximal obstructed colon can be expected (Figure 1H). If the stent is sitting slightly too proximal, a biopsy forceps can be inserted through the colonoscope and used to grasp the stent to manoeuvre it distally. In our experience, the stent over time can migrate distally. Post-deployment radiography is performed to confirm position.
Tips & pearls
- If biopsies are required, they should only be performed once the wire is in a confirmed position or after stent is deployed and in position to prevent bleeding from obscuring the view.
- It is not advisable to further dilate the stricture if it remains tight after SEMS deployment, given that the SEMS will continue to exert radial force and open the stricture.
Post-operative considerations and tasks
Patients are routinely kept for observation overnight to ensure resolution of colonic obstruction and to monitor for acute complications. An abdominal radiograph is taken the following day to confirm the position of the SEMS (Figure 1I). Upon discharge, patients are educated on dietary modification, specifically a low residue diet (minimal insoluble fibre) and laxatives to maintain a soft stool consistency.
Discussion
Endoscopic colonic SEMS insertion is a useful treatment option in the management of select patients with obstructing colorectal cancer. Due to the relatively infrequent nature of the procedure in daily practice overall, it is performed by only a proportion of the surgeons in our craft group to maintain technical proficiency.
Conclusions
We present the technical steps in performing colonic SEMS insertion in a detailed fashion, including several pitfalls that may be encountered and troubleshooting that may be required.
Acknowledgments
The authors would like to acknowledge the contributions of Dr. Alex Wong (MBBS, FRACS), Dr. Pravin Ranchod (MBChB, FRACS) and Dr. Ankur Sidhu (MBBS, FRACS) for their contributions to this paper.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-22-50/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Colorectal Endoscopic Stenting Trial (CReST) for obstructing left-sided colorectal cancer: randomized clinical trial. Br J Surg 2022;109:1073-80. [Crossref] [PubMed]
- van Hooft JE, Veld JV, Arnold D, et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy 2020;52:389-407. [Crossref] [PubMed]
- Cao Y, Gu J, Deng S, Li J, et al. Long-term tumour outcomes of self-expanding metal stents as 'bridge to surgery' for the treatment of colorectal cancer with malignant obstruction: a systematic review and meta-analysis. Int J Colorectal Dis 2019;34:1827-38. [Crossref] [PubMed]
- Clarke L, Abbott H, Sharma P, et al. Impact of restenting for recurrent colonic obstruction due to tumour ingrowth. BJS Open 2017;1:202-6. [Crossref] [PubMed]
- Köneş O, Kartal A, Akarsu M, et al. Colonic Stent Use in Patients With Malignant Flexure Tumors Presenting With Obstruction. JSLS 2019;23:e2018.00088.
Cite this article as: Wei MYK, Othman B, Chandra R, Balakrishnan V, An V. A detailed guide to endoscopic colonic stent insertion in obstructing colorectal cancer. Ann Laparosc Endosc Surg 2023;8:11.


