
Current applications of indocyanine green fluorescence in colorectal surgery: a narrative review
Introduction
The existing evidence indicates that up to one-third of patients undergoing colorectal surgery experience serious postoperative complications, which is a higher incidence than observed in other surgical specialties (1). Patients with complications from colorectal surgeries are at a greater risk for mortality, poor quality of life, and additional postoperative complications; 2% to 5.8% of those patients require a second operation (1). Improvements in surgical technique can have a positive impact on a patient’s length of stay, long-term outcomes, need for reoperations or discharge to higher levels of care—factors that ultimately affect the patient’s quality of life.
The regular use of indocyanine green (ICG) coupled with fluorescence angiography (FA) to assess tissue perfusion in colorectal surgery has recently been proposed as a feasible tool to help improve patient outcomes (1,2). ICG is a fluorophore dye used to visualize and assess in vivo organ perfusion when injected into the venous circulation of the patient intraoperatively. By observing the signal using a fluorescence imaging video system, surgeons can visualize and assess organ perfusion intraoperatively while making adjustments in real-time (3).
There are various intraoperative imaging modalities designed for determination of perfusion via ICG. Other fluorophore dyes can also be employed for a variety of purposes in the colorectal field. Methylene blue (American Regent, New York, NY, USA) and IRDye 800 CW (LI-COR Biosciences, Lincoln, NE, USA), in particular, have been employed for intraoperative ureter imaging (4). Methylene blue, however can cause serious side effects such as allergy, methemoglobinemia, and acute haemolysis. Also, the IRDye 800 CW is substantially more costly than the ICG (approximately ten-fold more expensive).
ICG intraoperative usage has become common in a variety of surgical specialties, including hepatobiliary, breast cancer, gastric cancer, and transplant surgery (5). Colorectal surgery has just lately begun to adopt this technique, particularly for the identification of perfusion in order to prevent anastomotic leakage (AL) (6). Using the best available data and the most cautious base case values, it has been shown that routine use of ICG-FA in colorectal surgery reduces costs (4). Existing evidence indicates a reduction in leak rate, therefore regular use of ICG-FA might be advisable (5).
We have reviewed the existent literature on both qualitative and quantitative assessment of bowel perfusion, tumour marking and sentinel lymph node detection, mapping of lymphatic drainage, as well as the identification of ureters. The goal is to delineate the recognized and potential benefits that intraoperative ICG dye use has in the colorectal surgical field, as well as the limitations and drawbacks that should be addressed to enhance widespread adoption. We prepared this article in accordance with the Narrative Review reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-22-84/rc).
Methods
We conducted a narrative review of the existing literature on the current applications of ICG-FA use during colorectal surgery and evaluated these applications in light of the reported technological limitation. In consultation with an information specialist from the Bracken Health Science Library of Queen’s University (Kingston, Ontario, Canada), we searched MEDLINE and Embase for articles published through March 30, 2023. Results were imported into Covidence for evaluation of abstracts and full-text. In addition, we conducted a Google Scholar search to identify relevant grey literature. The detailed search strategy and MeSH search terms are available in Table 1. Two study investigators (E.M. and J.U.) also manually searched the references of the included studies. Duplicate results were removed, and articles with their citations, titles, and abstracts were entered into Covidence for review management (7). All results were screened independently for abstract and full-text screening by two authors (E.M. and J.U.). Disagreements were resolved by consulting the senior author (A.C.M.).
Table 1
| Items | Specification |
|---|---|
| Date of search | 2022 to 2023/03/30 |
| Databases and other sources searched | MEDLINE, Embase |
| Search terms used | Colorectal surgery, colorectal surgery.mp, abdominal surgery, gastrointestinal surgery, colon cancer, indocyanine green, ICG.mp, fluorescence angiography, anastomosis leakage, anastomosis leak*.mp, quanti*.mp |
| Timeframe | Up to March 2023 |
| Inclusion criteria | English studies, all study types considered |
| Selection process | Included articles were selected independently and verified with co-authors for relevance |
Inclusion/exclusion criteria
Articles that discussed the use of ICG in the colorectal surgery setting were included. When a substantial corpus of literature was available, meta-analyses and randomized controlled trials (RCTs) were given preference. We excluded publications that were unavailable in the English language, conference abstracts without corresponding full manuscripts, case reports, case series, vignettes, and other minor studies, except in areas where literature was scarce. Our study inclusion process is detailed in Figure 1.
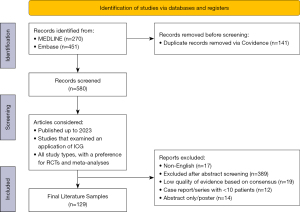
Results
Prevention of ALs
One meta-analysis study composed of RCTs that analyzed AL rates for ICG use in colorectal surgeries found that intraoperative ICG-FA was significantly protective against anastomotic leaks, but with a small magnitude of effect AL incidence decreased by 4% (8). Another study found that the use of ICG resulted in a 4.9% decrease in AL rates (9). A recent meta-analysis found that the use of intraoperative ICG led to a 4% decrease in AL rates, 4.3% decrease in reoperation rates, and 5.6% decrease in complication rates of patients undergoing colorectal surgeries for cancer (3). The doses of ICG dye used in the studies included in this meta-analysis ranged from 0.2 mg/kg or 2 mL to 10 mg or 0.5 mg/kg.
There have been other studies that showed significant decreases in AL rates, even with the qualitative use of ICG. The “A Study of Perfusion of Colorectal Anastomosis using Fluorescence Angiography” (FLAG) trial found that the use of ICG-FA (0.2 mg/kg) resulted in a 7.2% decrease in AL rates. This RCT focused mainly on patients receiving anterior resections, showing that intraoperative ICG-FA usage may play a larger impact in lowering AL rates for anastomoses involving the rectum (9). This is especially true for those formed less than 6 cm from the anal verge and for individuals with a body mass index (BMI) greater than 25 kg/m2 (10).
Another similar study examined the reductions in AL rates by subdividing papers based on the specific surgical procedure performed (Table 2). The subgroup analysis performed by the authors revealed that ICG use (dosages ranging from 0.1 to 0.5 mg/kg) to reduce AL rates was most effective when anastomoses involved the rectum or anus reconstruction. In contrast, ICG had no effect on AL rates when colonic anastomoses were performed. The group with low/ultra-low colorectal reconstructions had the greatest decrease in AL rates, whereas the cohort with colonic resections had the least decrease.
Table 2
| Surgery performed | No. of patients [studies] | ICG group AL rate (%) | Non-ICG group AL rate (%) | Difference (%) | Published year range |
|---|---|---|---|---|---|
| Rectal or anal anastomosis | 5,021 [20] | 3.5 | 9.4 | −5.9 | 2013–2021 |
| Anterior rectal resection | 4,367 [16] | 4.0 | 9.5 | −5.5 | 2013–2021 |
| Low/ultra-low anterior resection | 2,983 [14] | 4.1 | 11.1 | −7.0 | 2018–2020 |
| Low/ultra-low anterior resection (cancer only) | 2,391 [11] | 4.4 | 11.7 | −7.3 | 2018–2020 |
| Colectomy | 2,147 [5] | 1.7 | 5.0 | −3.3 | 2018–2020 |
| Left/sigmoid colectomy | 1,383 [4] | 2.0 | 6.7 | −4.7 | 2018–2020 |
| Right colectomy* | 626 [3] | 1.8 | 2.0 | −0.2 | 2018–2020 |
| Colorectal cancer | 4,241 [16] | 3.6 | 9.5 | −5.9 | 2013–2021 |
| Rectal cancer | 3,630 [13] | 3.4 | 9.5 | −6.1 | 2013–2021 |
Table created from data obtained from Trastulli et al. (11). *, there was no significant difference in AL rates between the ICG group and non-ICG group for patients undergoing right colectomy. AL, anastomotic leakage; ICG, indocyanine green dye.
The bulk of research on the prevalence of AL have been on rectal and left-sided colectomies. A new meta-analysis sought to ascertain the impact of ICG in lowering complications and AL rates in right-sided surgeries (12). The authors found that the use of ICG resulted in an AL rate of 1% for right-sided colectomies (n=144) and an AL rate of 3% for left-sided colectomies (n=371). They concluded that intraoperative ICG use for right-sided colectomies reduced AL rates by half. However, this result lacks statistical significance given the small sample size for this procedure. Thus, additional research is required to completely elucidate the advantages of ICG use in right-sided colectomies. These findings support what surgeons have observed and reported anecdotally in recent years. The more distal the bowel continuity restoration, the more advantageous the use of ICG (11).
Quantification
Conflicting results may be ascribed to the subjective nature of the qualitative ICG measure, which is used to supplement clinical judgment and reasoning. In order to evaluate parameters such as perfusion and environmental noise, surgeons must make in situ determinations of fluorescence intensity. Due to the subjective nature of ICG-FA interpretation, the external validity of any single institution study is compromised. Therefore, there is substantial interest in standardizing the use of ICG fluorescence prior to the technique’s widespread adoption in colorectal surgery (13-16). ICG utilization is hampered by literature limitations and discrepancies.
There are several technical aspects of fluorescence imaging that limit the reliability and reproducibility of quantification of ICG dye to assess perfusion (17). First, the intensity of the dye is inversely correlated to the distance of the dye to the detector. Thus, detectors that are placed further away from the ICG source will show less intensive fluorescence signals than those detectors placed close by. In addition, the dynamic process of the fluorophore dye results in distribution of some of the dye to ischemic zones over time, rather than solely being contained in regions that are adequately perfused. This ultimately results in underestimates of the extent of ischemic zones (17).
Quantifying ICG dye fluorescence
D’Urso et al. have developed a fluorescence-based enhanced reality (FLER) system that analyzes and displays the dynamic evolution of the fluorescent signals in order to overcome the aforementioned limitations of fluorescence detection (17). This allows surgeons to obtain proximal and distal FLER analysis and mark regions of interests with a mean time of 7.8±3.34 min, and the quantified values were well correlated with biomarkers of perfusion. This illustrates that the FLER program may be used to provide precise quantification of fluorescence imaging as well as standardized data in order to restrict the influence of ICG on anastomotic problems.
Another study looked at the factors influencing intensity and pattern of ICG-FA (dosage of 7.5 mg) in order to predict AL (18). The researchers performed a retrospective analysis of patients who underwent elective surgery for colorectal cancer (n=70) and found that the time to half maximal intensity of fluorescence was the most robust predictor of AL, and quantification of this parameter allowed identification of patients that were at high or low risk of AL.
A retrospective analysis of 112 patients who underwent laparoscopic surgery for left-sided colorectal cancers evaluated whether quantitative assessment of intestinal perfusion (ICG dosage of 5 mg) could predict adverse outcomes of colorectal surgery (19). The authors used recorded video images to retrospectively create time-fluorescence intensity curves to analyze four parameters (fluorescence difference between maximum and baseline fluorescence, time from first fluorescence increase to maximum, time from first fluorescence increase to half of maximum, and the slope of the curve) between the group of patients that had AL versus those that did not. The authors found that the fluorescence difference between maximum and baseline was the most indicative of AL.
A similar prospective study was also performed to evaluate the how the quantitative measurement of ICG imaging (dosage of 5 mg/2 mL) reduced the risk of AL (20). The researchers found that in all non-AL cases (n=19), the fluorescence around the anastomosis emerged by 30 seconds after the ICG injection. In contrast, the researchers found that for the patients with AL cases (n=3), the fluorescence did not emerge until 72 seconds after the ICG injection. Thus, the researchers found that the time of emergence of fluorescence could be used as a sensitive predictor of AL. However, this study was limited by its small number of participants, and further studies can further elucidate the benefits of using other quantitative parameters involving fluorescence emergence.
Quantification studies show that ICG may be utilized to provide objective assessments of sufficient perfusion during colorectal surgery. Due to the small number of studies conducted thus far, it is problematic to infer that measurement of ICG dye results in decreased rates of AL. Further research with larger sample sizes on the measurement of ICG-FA in colorectal procedures are needed to demonstrate the technique’s importance in lowering AL rates.
Lymph node detection and lymphatic marking
The first lymph node to receive discharge from a lesion and surrounding region is the sentinel lymph node. Sentinel lymph node detection and biopsy can give significant clinical and prognostic information and is frequently performed in conjunction with standard tumour removal. Sentinel lymph node detection is therefore an essential step in the staging of any primary malignancy, including colorectal cancer. Several studies have estimated the predictive capability of ICG paired with near-infrared fluorescence in detecting positive sentinel lymph nodes in primary colorectal cancer (21,22). In their systematic review and meta-analysis, Emile and colleagues found that across 12 studies (248 patients), intraoperative ICG use (concentrations ranging from 0.5 to 5 mg/mL and doses ranging from 0.2–5 mL) had a pooled sensitivity and specificity of 71.0% and 84.6%, respectively (21). In their recent meta-analysis including 227 patients across 8 studies, Ankersmit et al. found that intraoperative ICG use (concentration of 2.5 mg/mL) had sensitivity of 63% in detecting positive sentinel lymph nodes (22).
There is often interest in identifying and removing the entire lateral pelvic lymph node compartment due to their role in local cancer recurrence, especially in low rectal cancers (6). On top of its role in detection of positive sentinel lymph nodes, ICG has also been shown to be useful in identifying lymphatic drainage to facilitate total lateral lymph node dissection (6). Several studies have outlined the benefit of using ICG to map out lymphatic drainage during colorectal surgery (13,23,24). One study conducted in 2019 found that patients that received ICG (concentration of 0.1 mg/mL) during rectal cancer surgery to facilitate lymph node visualization had significantly less intraoperative bleeding and a larger number of lymph nodes harvested compared to patients that did not receive ICG (23). Another small study found that when ICG and fluoroscopy was applied during colorectal neoplasia resection, both sentinel nodes and non-sentinel nodes could be identified and differentiated in situ for all 14 patients included (24). Finally, Ushijima and colleagues found that the use of ICG (dosage of 0.2 to 0.3 mL) allowed them to clearly and accurately identify regional lymphatic drainage in 75.4% of patients that received ICG during elective colorectal cancer surgery (13).
These studies have highlighted the feasibility of using ICG for detection of sentinel lymph nodes in colorectal cancer. However, evidence remains limited and additional studies—including prospective and randomized trials—are warranted to address the real clinical utility of this tool.
Tumour marking
With minimally invasive surgery becoming the standard for managing colorectal cancer, the accurate identification and localization of a tumor has become crucial. Accurate identification and localization of tumours allows surgeons to precisely resect margins that would remove the tumour while maximizing functional sparing of healthy tissue. This identification and localization of a tumour can be augmented with other intraoperative navigation methods (14). The use of intraoperative ICG has shown potential in accurate and reliable identification of tumours for colorectal cancer surgeries (15).
One study used this technique for 165 patients undergoing laparoscopic colorectal surgery in order to optimize the concentration of ICG dye that would result in the most sensitive detection of colorectal tumours (16). The authors demonstrated ICG fluorescence marking with an ICG dosage of 0.5 mg to be a reliable and reproducible method for tumor site marking as it allowed the tumors to be visible for all 165 patients when injected at least 6 days prior to the laparoscopic surgery. The added benefits of using small injection volumes include reducing the risk of the ICG dye expanding to other areas, and/or leaking into the peritoneum, preventing misidentification of the tumour sites.
A novel technique utilizing ICG-coated endoscopic clips has been proposed, to overcome some of the limitations of conventional utilization of ICG (25). These endoscopic clips coated with ICG dye are placed at the lesion site two days prior to the surgery (to prevent any bowel distension due to intraluminal air insufflation), and the location of the tumour is determined by detecting the fluorescence signal of the ICG-coated endoscopic clips through the intestinal wall using near-infrared laparoscope. The ICG-coated clips were observed intraoperatively in 22 of the 27 instances, according to the investigators. The ICG-coated clipping process took an average of 5.3 minutes, and there were no incidences of treatment-related problems. The 5 cases where the ICG-coated clips were not visible might be attributed to the colon being covered with dense adipose tissue or mesorectum, obscuring the clips’ sight due to the fluorophore’s 10 mm tissue penetration. To address this, increased detection rates may be explored. Yet, the authors proved that ICG-coated chips have the potential to be used as a safe and viable way to improve intraoperative ICG usage for tumour localisation in colorectal procedures.
Another study utilized artificial intelligence for determination of colorectal liver metastases (26). The researchers administered a small dose of ICG (0.05 mg/kg) in 25 patients with liver surface lesions that were undergoing surgical treatment. They used the results of the perfusion profiles to train a machine learning classifying. This algorithm was shown to correctly identify 97.2% of colorectal liver metastases (n=132) and 100% of all benign lesions (n=6).
Ureter identification
Another possible complication of colorectal surgery is ureteric injury; it has been established that the insertion of stents do not necessarily prevent injuries, but they do aid in the diagnosis of injuries if they do occur. Identification and preservation of the ureters are commonly viewed as critical to avert difficulties, particularly in patients with acute diverticulitis, endometriosis, locally advanced malignancies, obesity, and Chron’s illnesses, where anatomical distortion may make localization difficult (27). In colorectal surgery, ICG has emerged as a potential method for ureter detection. Several studies have demonstrated that it is useful in minimizing ureteric damage (27,28).
Another research looked at the effectiveness and procedure-related problems of adult patients who received intraoperative ureter identification by way of ICG during their colorectal surgery (28). They found 142 patients, with only one sustaining intraoperative ureteric damage. ICG is eliminated through the liver, therefore it does not normally enter the urinary system; for the purpose of ureteric identification, it needs to be instilled into the ureter, most often via stents. In their investigation, Garoufalia et al. discovered that using intraluminal ureteric ICG resulted in an additional 12.8 minutes of operating time (28). There are no comparable studies included in this research study, limiting its total efficacy.
One study (27) conducted a systematic evaluation of 12 trials to investigate the efficacy of ICG usage in minimally invasive colorectal surgery. Five of the 12 investigations were retrospective, 2 were case studies, and 5 were prospective. The authors discovered that just three of the 822 individuals who underwent ureter identification suffered from ureteric damage. No comparable studies were included in this study, further studies are required to confirm its usefulness.
Nevertheless, the use of ICG for identification of ureters is a promising technique for prevention of ureteric injuries.
ICG complications
It is important to note that the use of ICG may lead to increased incidence of other complications among specific sub-populations (8). The rate of ileus has been shown to be significantly higher for patients that underwent colorectal surgeries with ICG compared to those that did not in seven studies from Western countries (8). However, the rate of ileus was significantly lower in the ICG group compared to the non-ICG group in three studies from East Asia (8).
There may be several potential disadvantages of using ICG for tumour marking. A drawback of using ICG dye for tumour localisation is that the usual ICG dosage used for angiography causes interference through staining of adjacent tissues, making it difficult to discern the tumour. As a result, ICG concentrations must be tailored for their intended use (29). One study has shown that approximately 2–3% of the injected dye is at risk of spreading into the peritoneal space, limiting the technique’s sensitivity (30). In addition, another study demonstrated that ICG dye injected into patients more than two days prior to the surgery resulted in the site being identified in only 40% of cases (31).
Discussion
This review summarizes the most recent studies outlining the different applications of ICG in colorectal surgery. Intraoperative use of ICG allows surgeons to subjectively estimate ‘real-time perfusion status of patients’ colons, monitor for any ischemic changes at the colonic anastomosis sites, and make real-time changes to reduce potential complications of the surgery. Current studies have mostly focused on qualitative assessments of ICG fluorescence, which has been shown to reduce the risk of ALs, although with a small overall magnitude.
In the existing studies a wide range of ICG dosages and times between administration and dye evaluation to assess perfusion have been used (21). There is also variation in surgeons’ capacity to interpret perfusion using ICG dye (22). Various studies have used various definitions for AL, ranging from any radiologic or endoscopic disruption to clinically suspected anastomotic dehiscence (23). Standardization of the definition of AL is necessary for comparing studies and elucidating the benefits of intraoperative ICG use to reduce AL. Existing research indicates that ICG-FA has the ability to minimize the rate of AL in colorectal procedures. More well-designed randomized controlled studies are needed to confirm the effectiveness of intraoperative ICG-FA in avoiding such complications and reoperation. To resolve these knowledge gaps, two RCTs (ICG-COLORAL; NCT03602656 and InTACT trial; ISCRN 13334746) are actively enrolling patients. Moreover, the “Anastomotic Leakage and Value Of Indocyanine green dye in Decreasing leakage rates” (AVOID) study (an RCT) has been proposed to further elucidate the benefits of intraoperative ICG use in reducing AL rates associated with colorectal procedures (24).
Additional research is required to determine the effect of various anastomotic techniques on the incidence of AL. For instance, anti-peristaltic end-to-end anastomosis with a linear stapler and double stapling techniques with circular staplers in sigmoid resections are associated with their own risks of AL and must be studied separately to determine the effects of ICG fluorescence on reducing AL (20). To determine the usefulness of quantitative measurements of ICG fluorescence in minimizing the risk of ALs, well-designed RCTs are required. Guidelines for consensus in the use of ICG fluorescence are required for broad adoption of this technique in the field of colorectal surgery.
Studies incorporated ICG for lymph node detection and lymphatic marking during colorectal surgeries. These studies show that there is good predictive capability of ICG in detecting positive sentinel lymph nodes in primary colorectal cancer and identifying lymphatic drainage to aid in total lymph node dissections. Tumor identification and localization via ICG has also been shown to be adequate in resecting margins of tumors to remove cancerous tissue while maximizing the functional sparing of healthy tissue.
There is also progress in exploiting ICG for other purposes. There are still incidences of ureteric damage during minimally invasive colorectal procedures, for example, and ICG dye has showed promise in enabling for ureter imaging to minimize such issues. It has also demonstrated promise in locating the transanal complete mesorectal dissection plane to help with mesorectal excisions and prevent urethral damage (32).
There is a growing body of evidence for intraoperative ICG-FA use to be incorporated into the standard of care of colorectal surgeries.
There are inherent limitations of a narrative review in contrast to a systematic review or meta-analysis. Our study did not impose explicit, predetermined criteria for article selection and while included articles were assessed for validity, they were not subject to rigorous evaluation.
Conclusions
As further studies and RCTs are conducted to overcome the technical constraints of this technique as detailed in numerous existing articles, intraoperative ICG usage offers enormous potential in improving overall patient outcomes for patients undergoing colorectal surgery.
Acknowledgments
We would like to thank Mrs. Angelique Roy, the information specialist at Queen’s University, Bracken Health Sciences Library, for refining our search strategy.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-22-84/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-22-84/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dinallo AM, Kolarsick P, Boyan WP, et al. Does routine use of indocyanine green fluorescence angiography prevent anastomotic leaks? A retrospective cohort analysis. Am J Surg 2019;218:136-9. [Crossref] [PubMed]
- Shen R, Zhang Y, Wang T. Indocyanine Green Fluorescence Angiography and the Incidence of Anastomotic Leak After Colorectal Resection for Colorectal Cancer: A Meta-analysis. Dis Colon Rectum 2018;61:1228-34. [Crossref] [PubMed]
- Liu D, Liang L, Liu L, et al. Does intraoperative indocyanine green fluorescence angiography decrease the incidence of anastomotic leakage in colorectal surgery? A systematic review and meta-analysis. Int J Colorectal Dis 2021;36:57-66. [Crossref] [PubMed]
- La Regina D, Di Giuseppe M, Lucchelli M, et al. Financial Impact of Anastomotic Leakage in Colorectal Surgery. J Gastrointest Surg 2019;23:580-6. [Crossref] [PubMed]
- Liu RQ, Elnahas A, Tang E, et al. Cost analysis of indocyanine green fluorescence angiography for prevention of anastomotic leakage in colorectal surgery. Surg Endosc 2022;36:9281-7. [Crossref] [PubMed]
- Peltrini R, Podda M, Castiglioni S, et al. Intraoperative use of indocyanine green fluorescence imaging in rectal cancer surgery: The state of the art. World J Gastroenterol 2021;27:6374-86. [Crossref] [PubMed]
- Covidence systematic review software [Internet]. Melbourne, Australia: Veritas Health Innovation. Available online: www.covidence.org
- Zhang W, Che X. Effect of indocyanine green fluorescence angiography on preventing anastomotic leakage after colorectal surgery: a meta-analysis. Surg Today 2021;51:1415-28. [Crossref] [PubMed]
- Chan DKH, Lee SKF, Ang JJ. Indocyanine green fluorescence angiography decreases the risk of colorectal anastomotic leakage: Systematic review and meta-analysis. Surgery 2020;168:1128-37. [Crossref] [PubMed]
- Arezzo A, Bonino MA, Ris F, et al. Intraoperative use of fluorescence with indocyanine green reduces anastomotic leak rates in rectal cancer surgery: an individual participant data analysis. Surg Endosc 2020;34:4281-90. [Crossref] [PubMed]
- Trastulli S, Munzi G, Desiderio J, et al. Indocyanine green fluorescence angiography versus standard intraoperative methods for prevention of anastomotic leak in colorectal surgery: meta-analysis. Br J Surg 2021;108:359-72. [Crossref] [PubMed]
- Mok HT, Ong ZH, Yaow CYL, et al. Indocyanine green fluorescent imaging on anastomotic leakage in colectomies: a network meta-analysis and systematic review. Int J Colorectal Dis 2020;35:2365-9. [Crossref] [PubMed]
- Ushijima H, Kawamura J, Ueda K, et al. Visualization of lymphatic flow in laparoscopic colon cancer surgery using indocyanine green fluorescence imaging. Sci Rep 2020;10:14274. [Crossref] [PubMed]
- van Manen L, Handgraaf HJM, Diana M, et al. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J Surg Oncol 2018;118:283-300. [Crossref] [PubMed]
- ASGE Technology Committee. Endoscopic tattooing. Gastrointest Endosc 2010;72:681-5. [Crossref] [PubMed]
- Satoyoshi T, Okita K, Ishii M, et al. Timing of indocyanine green injection prior to laparoscopic colorectal surgery for tumor localization: a prospective case series. Surg Endosc 2021;35:763-9. [Crossref] [PubMed]
- D'Urso A, Agnus V, Barberio M, et al. Computer-assisted quantification and visualization of bowel perfusion using fluorescence-based enhanced reality in left-sided colonic resections. Surg Endosc 2021;35:4321-31. [Crossref] [PubMed]
- Gomez-Rosado JC, Valdes-Hernandez J, Cintas-Catena J, et al. Feasibility of quantitative analysis of colonic perfusion using indocyanine green to prevent anastomotic leak in colorectal surgery. Surg Endosc 2022;36:1688-95. [Crossref] [PubMed]
- Wada T, Kawada K, Takahashi R, et al. ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 2017;31:4184-93. [Crossref] [PubMed]
- Hayami S, Matsuda K, Iwamoto H, et al. Visualization and quantification of anastomotic perfusion in colorectal surgery using near-infrared fluorescence. Tech Coloproctol 2019;23:973-80. [Crossref] [PubMed]
- Emile SH, Elfeki H, Shalaby M, et al. Sensitivity and specificity of indocyanine green near-infrared fluorescence imaging in detection of metastatic lymph nodes in colorectal cancer: Systematic review and meta-analysis. J Surg Oncol 2017;116:730-40. [Crossref] [PubMed]
- Ankersmit M, Bonjer HJ, Hannink G, et al. Near-infrared fluorescence imaging for sentinel lymph node identification in colon cancer: a prospective single-center study and systematic review with meta-analysis. Tech Coloproctol 2019;23:1113-26. [Crossref] [PubMed]
- Zhou SC, Tian YT, Wang XW, et al. Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer. World J Gastroenterol 2019;25:4502-11. [Crossref] [PubMed]
- Cahill RA, Anderson M, Wang LM, et al. Near-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasia. Surg Endosc 2012;26:197-204. [Crossref] [PubMed]
- Lee DW, Sohn DK, Han KS, et al. Promising Novel Technique for Tumor Localization in Laparoscopic Colorectal Surgery Using Indocyanine Green-Coated Endoscopic Clips. Dis Colon Rectum 2021;64:e9-e13. [Crossref] [PubMed]
- Hardy NP, Epperlein JP, Dalli J, et al. Real-time administration of indocyanine green in combination with computer vision and artificial intelligence for the identification and delineation of colorectal liver metastases. Surg Open Sci 2023;12:48-54. [Crossref] [PubMed]
- Mazzarella G, Muttillo EM, Picardi B, et al. Real-Time Intraoperative Ureteral Identification in Minimally Invasive Colorectal Surgery: A Systematic Review. J Laparoendosc Adv Surg Tech A 2022;32:627-33. [Crossref] [PubMed]
- Garoufalia Z, Wexner SD. Ureter identification utilizing indocyanine green (ICG) imaging in colorectal surgery: a systematic review of the literature. Mini-invasive Surg 2022;6:51. [Crossref]
- Son GM, Ahn HM, Lee IY, et al. Multifunctional Indocyanine Green Applications for Fluorescence-Guided Laparoscopic Colorectal Surgery. Ann Coloproctol 2021;37:133-40. [Crossref] [PubMed]
- Hammond DC, Lane FR, Mackeigan JM, et al. Endoscopic tattooing of the colon: clinical experience. Am Surg 1993;59:205-10. [PubMed]
- Lee SJ, Sohn DK, Han KS, et al. Preoperative Tattooing Using Indocyanine Green in Laparoscopic Colorectal Surgery. Ann Coloproctol 2018;34:206-11. [Crossref] [PubMed]
- Keller DS, Ishizawa T, Cohen R, et al. Indocyanine green fluorescence imaging in colorectal surgery: overview, applications, and future directions. Lancet Gastroenterol Hepatol 2017;2:757-66. [Crossref] [PubMed]
Cite this article as: Uppal JS, Meng E, Caycedo-Marulanda A. Current applications of indocyanine green fluorescence in colorectal surgery: a narrative review. Ann Laparosc Endosc Surg 2023;8:18.


