
Colon cancer: is the robot a better option?
Introduction
Minimally invasive surgery (MIS) approaches in colorectal cancer have been widely adopted to minimize morbidity and enhance recovery. The first descriptions of laparoscopic colon resection were reported in the early 1990s (1,2), with a steady increase during the last 30 years, representing now more than 50% of the colectomies around the world (3).
Several landmark studies have shown equivalence and/or non-inferiority between open and laparoscopic colorectal resections (4-6) for both short-term and oncological outcomes. The main advantages of MIS techniques include an earlier return of bowel function and oral intake, shorter length of in-hospital stay, and diminished postoperative pain.
In the last 2 decades, robotic platforms have made a breakthrough in MIS surgery, especially in anatomically difficult areas such as the pelvis. Robotic platforms are well-suited for difficult pelvic surgery due three-dimensional visualization, degree of articulation of instruments, precise movements, and better ergonomics.
Since 2010 onward, robotic surgery use has been steadily increasing, with an 11% annual increase in colorectal resections (7). Despite the aforementioned benefits, there is no clarity on the advantages of the use of robotic platforms for colon cancer surgery. The aim of the present editorial comment is to evaluate the benefits and disadvantages of robotics for colon cancer surgery.
Overview of robotic colon surgical resection
Patient setup and trocar disposition
In our personal experience, robotic colectomy is frequently performed with a single docking technique using the DaVinci® Xi system, with the robotic cart docked over the right side (if it is a right colectomy) or left side (if it is a left colectomy) of the patient. Patients are usually secured with both arms tucked and a foam mattress directly under the patient is used to prevent sliding during the operation. Routinely we use a urinary catheter that is usually removed after 24 hours.
We recommend induced pneumoperitoneum using a Veress needle in the left upper quadrant (Palmer’s point). For most of the surgeries, a three-arm technique is used, with an assistant port to help mobilize the small bowel. A 12-mm robotic stapler port may be installed for colon section and/or intracorporeal anastomosis (ICA). If available, an operating table with integrated motion is highly recommended, allowing to reposition the patient while the surgical robotic arms are still docked. In the right hand, usually a monopolar scissor or vessel sealer is usually used, while a fenestrated bipolar forceps is used commonly in the left hand. A ProGrasp™ or Cadiere forceps can be used via a 4th arm for retraction in special cases when needed.
Right colon cancer
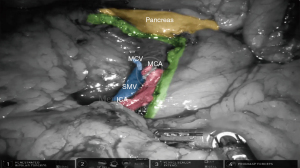
In the robotic approach, we frequently used a retrocolic tunnel using a medial-to-lateral approach. This tunnel should be dissected directly below the ileocolic vessels that are obtained after lateral traction of them. Alternatively, a bottom-up approach can be performed starting from the inferior aspect of the cecum. Before any vascular division, both the duodenum and right ureter should be properly identified. A central vascular ligation of the ileocolic vessels should be routinely performed. When a complete mesocolic excision (CME) is performed, the superior mesenteric vein or artery should be dissected to identify the origin of the ileocolic and middle colic vessels (Figure 1). In addition, a central ligation may require control of the gastrocolic trunk, with an optional preservation of the right gastroepiploic vein.
Transverse colon and flexures
The main landmark during a robotic transverse colon cancer resection is defined by the middle colic vessels as the central vascular pedicle between colonic flexures (hepatic and splenic). The greater omentum should be opened and/or resected for the proper mobilization of the any of the flexures (hepatic and/or splenic). Careful anatomical knowledge is critical, since the presence of adhesions in relationship with the anterior surface of the transverse colon may be different between patients, making the surgical approach more challenging. For a central ligation of the middle colic vessels, access to the lesser sac is mandatory. A complete mobilization of both flexures is needed for an oncological resection of the transverse mesocolon. The root of the transverse mesocolon can be identified by anterior and superior traction of transverse colon, creating a fold that separates the mesentery of the small bowel from the transverse mesocolon.
Splenic flexure takedown is one of the most difficult approaches of MIS in colon cancer. Using a standardized approach, we first identify the inferior mesenteric vein right below the inferior pancreatic border at the angle of Treitz. A retrocolic tunnel is dissected just below the inferior mesenteric vein, gaining access to the anterior aspect of the pancreas and allowing it to be dissected free from medial to lateral. Lateral attachments of the left colon are opened until the 2 planes converge using a medial-to-lateral approach. Once a complete mobilization of the colon is achieved, a central vascular ligation should be performed in case of tumors located in the distal transverse/splenic flexure, controlling both left colic artery and the left branch of the middle colic vessels at their respective origins.
Left and sigmoid colon
In our practice, a robotic left colon cancer resection starts with identification of the inferior mesenteric vein at the level of the ligament of Treitz. At this level, a medial-to-lateral approach is performed beneath the inferior mesenteric vein, lifting the mesocolon over the left ureter, left gonadal vessels and Gerotas’s fascia. For central ligation in a sigmoid resection, we routinely open the peritoneum over the sigmoid mesocolon right above the left common iliac artery until the origin of the inferior mesenteric artery is identified at the aorta. A similar approach is needed for descending colon cancer, but a complete splenic flexure takedown may be needed to obtain proper oncological margins and a tension-free anastomosis.
Potential benefits of robotic platforms for colon cancer resections
Clinical and oncological outcomes
When compared to laparoscopy, robotic surgery results in similar or shorter postoperative lengths of in-hospital stay, similar perioperative morbidity/anastomotic leak rates and lower rates of conversion to open surgery (8,9). Conversion to open resection may result in increased postoperative complication rates, higher costs, delays in time to initiation of systemic neoadjuvant/adjuvant chemotherapy which can finally have an impact on overall and disease-free survival (10).
However, is it our opinion that the true potential benefit of robotic platforms in colon cancer is defined by its use in more difficult or meticulous anatomical dissections such as CME in right colon cancer, where delicate vascular dissection and visualization is improved when compared to laparoscopy.
The principle underlying a mesocolic-oriented dissection in colon cancer surgery is the intact mobilization of the associated mesocolon, allowing a vascular central ligation with the complete removal of the lymph nodes contained within. The resected mesenteric area, lymph node harvest and mesocolic plane of excision rates are consistently higher in patients who have undergone CME as compared to conventional surgery, thereby increasing the nodal yield which may improve survival in colon cancer.
Retrospective series have shown that robotic CME is safe and feasible, with low morbidity, especially when potential vascular injury is considered (Table 1).
Table 1
| Author | Type of study | N | Conversion | Complications | Anastomotic leak | Mortality | Nº of LN removed | OS | DFS |
|---|---|---|---|---|---|---|---|---|---|
| Spinoglio, 2019 (11) | Retrospective | 202 (100 robotic) | 0% robotic vs. 6.9% lap | 27.7% robotic vs. 33.6% lap | 1% | 1% | 28.2 robotic vs. 30.2 lap | 77% robotic vs. 7% lap (5 years) | 85% robotic vs 83% lap (5 years) |
| Yozgatli, 2019 (12) | Prospective | 96 (35 robotic) | 0 | 29% robotic vs. 25% lap | 0 | NR | 41 robotic vs. 33 lap |
NR | NR |
| Yang, 2019 (13) | Retrospective | 66 | 1.5% | 7.6% | 0 | 0 | 32 | NR | NR |
| Bae, 2019 (14) | Retrospective | 43 | 0 | 21% | 2.3% | 0 | 29 | 93.6% (2.2 years) | 81.1% (2.2 years) |
| Park, 2019 (15) | RCT | 70 (35 robotic vs. 35 lap) | 0 | 17.1% robotic vs. 20% lap | 1% robotic vs. 0% lap |
0 | 29.9 robotic vs. 30.8 lap | 91.1% robotic vs. 91% lap | 77.4% robotic vs. 83.6% lap |
| Ramachandra, 2020 (16) | Retrospective | 52 | 3.84% | 11.5% (Clavien-Dindo >3) |
1.92% | 0 | 28 | NR | NR |
| Ceccarelli, 2021 (17) | Retrospective (26 robotic) | 55 | 0 | 46.2% robotic vs. 27.6% lap | 0% robotic vs. 3.4% lap | 0 | 20.4 robotic vs. 18.5 lap | NR | NR |
| Siddiqi, 2021 (18) | Retrospective | 77 | 0 | 11.7% | 0 | 0 | 30 | 94% (3 years) | 94% (3 years) |
| Bianchi, 2021 (19) | Retrospective | 161 | 3.7 | 16.1% | 0.6% | 0 | 21.9 | NR | NR |
| Khan, 2021 (20) | Retrospective | 40 robotic | 0 | 15% | 2.5% | 0 | 29 robotic vs. 18 lap |
90% (3 years) | 99% (3 years) |
CME, complete mesocolic excision; LN, lymph nodes; OS, overall survival; DFS, disease free survival; NR, not reported; RCT, randomized control trial.
In a published experience from Memorial Hospital (21), the robotic approach in right colon cancer produced a higher lymph node yield compared to other approaches such as open and laparoscopic (P<0.01), and a higher lymph node to length of surgical specimen ratio compared to open right colectomy (P<0.01). Further, in a recent large retrospective study from the same institution that included 2,398 patients that underwent non-metastatic primary colon cancer resections, robotic colon cancer was associated with higher lymph node yield and radicality of mesenteric resection, but no difference in overall survival and disease-free survival was observed when compared against open and/or laparoscopic approach (22).
Despite all of these data, the rationality of CME in right colon cancer may lie in a standardization of surgical technique, with some possible advantages of the robotic platform above other MIS techniques (23). In terms of oncological outcomes, we still await prospective trial data to inform the potential benefits and more data from randomized controlled trials should be available before advocating its widespread use—especially in low-volume centers without sufficient expertise.
ICA versus extracorporeal anastomosis (ECA)
One of the main advantages of the robotic platforms is the capacity to simplify more difficult MIS maneuvers such as intracorporeal suturing, specifically for ICA. In a recent systematic review that included only 5 randomized control trials, laparoscopic ICA was associated with a faster recovery of bowel function and lower rates of surgical site infections when compared with ECA (24). Similarly, in a multicenter observational trial across 20 institutions, robotic and laparoscopic ICA were associated with significant improvements in conversion rates, return of bowel function, and shorter hospital stay (25). Additionally, robotic ICA was associated with lower 1-year estimated incisional hernia rate than robotic ECA (2% vs. 12%, respectively) (26).
A retrospective review of 2 Italian hospitals showed that robotic right colectomy with ICA was superior to conventional laparoscopic right colectomy with ECA in terms of recovery outcomes such as shorter length of stay (27). However, no differences were observed when the comparison was against laparoscopic right colectomy with ICA. In a single series of 88 patients, robotic right colectomy with ICA had a shorter operative time during a standardized intracorporeal reconstruction when compared with the laparoscopic approach (28).
Both laparoscopic and robotic ICA have shown advantages in patient recovery and aesthetics, with robotic platforms making intracorporeal sutures simpler in a minimally invasive setting.
Learning curve
MIS learning curve has been studied for both laparoscopic and robotic colorectal resections. In a single-center case-mix adjustment analysis (29) that included conversion rate, reoperations, and readmissions, a learning curve of 55 cases for right-sided colonic resections versus 62 cases for left-sided resection was demonstrated using cumulative sum (CUSUM) analysis. The authors also determined that median operative time declined after gaining operative experience. However, surgical techniques such as ICA and CME may prolong learning curves especially in right-sided colon cancer.
Robotic right colectomy with ICA learning curve has been estimated at 44 cases according to a single center experience (30). In addition, a comparative analysis from robotic versus laparoscopic right colon cancer resections performed by surgical fellows with minimal experience in minimally invasive colorectal resections, the drop in operative time was seen earlier in the robotic group (16 vs. 25 cases, respectively) (31).
Overall, it seems that the learning curve for colon cancer resections is shorter and easier using robotic platforms versus a laparoscopic approach. This is of key importance for education of upcoming surgical trainees, considering that data on current robotics users has shown that general surgeons who have less than 10 years of post-training experience, board-certified colorectal surgeons and high-volume surgeons are more likely to be performing robotic surgery (32).
Disadvantages of robotic platforms for colon cancer resections
Operative time and cost
Operative time has been classically described as longer than laparoscopic approach. Recent pooled analysis has showed that robotic surgery adds around 51 minutes to operative time, which may be associated with an increase in morbidity and procedure-related cost. However, it has been shown that operative time with a robot can be similar to laparoscopy in specialized centers with high volume robotic cases. In our experience, operative time in robotic procedures has been decreased especially due dedicated personnel, widespread use of robotic approach and use of DaVinci® Xi system which allows a multi-quadrant approach without the need of re-docking.
The cost of robotic platforms is a common discussion point. Robotic systems initially require a large investment (33), but also adds ongoing maintenance and repair, the use of reusable instruments, as well as training expenses for the surgical team that is involved in its use. In addition, longer operative times and related costs have raised doubts about the value of this technology and how healthcare resources should be allocated. Approximately 10 years ago, it was estimated that robotics may add up to $3,500 per surgical procedure, adding nearly $2.5 billion annually to healthcare costs (33). Using data from national databases, several studies found that use of the robot was associated with an increased cost of approximately $2,000–5,000 per procedure when compared to laparoscopy (34). Robotic technology cost, however, is likely still related to surgical volume. A retrospective analysis using a national inpatient database showed that low-volume robotic colorectal surgeons were associated with higher rates of postoperative complications (P<0.0001), higher surgical-related costs (P<0.001), and longer in-hospital stay (P<0.001). A recent meta-analysis concluded that in centers with an annual volume of less than 10 cases having multiple surgeons performing robotic procedures led to worse outcomes (35). These findings should support the use of robotic technology in high-volume centers, potentially improving outcomes and reducing the costs associated with robotic surgery where the initial investment has been made and efforts can be focused on reducing costs and charges for consumables and reusable equipment.
Conclusions
Robotic colon cancer resection can be performed safely, with favorable short-term postoperative and oncologic outcomes and addressing some of the limitations of laparoscopy. However, robotic colectomy is associated with longer operative times and higher costs than laparoscopic colectomy.
In our experience, the use of robotic platforms in oncological colon cancer surgery has improved the ability to achieve better resections and offers the advantages of a minimally invasive approach in complex oncological resections. Robotic colectomy offers many advantages to the surgeon, but efforts should be made to reduce operative time and cost in a safe manner in centers where robotic technology has been adopted.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by Guest Editor (Alessandro Fichera) for the series “Minimally Invasive Colorectal Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-16/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-16/coif). The series “Minimally Invasive Colorectal Surgery” was commissioned by the editorial office without any funding or sponsorship. JJS reports that he has served as a clinical advisor for Foundation Medicine Inc. [2022], and he has been paid as a consultant and speaker for Johnson and Johnson Inc. [2022] and GlaxoSmithKline plc. [2023]. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991;1:144-50. [PubMed]
- Monson JR, Darzi A, Carey PD, et al. Prospective evaluation of laparoscopic-assisted colectomy in an unselected group of patients. Lancet 1992;340:831-3. [Crossref] [PubMed]
- Fox J, Gross CP, Longo W, et al. Laparoscopic colectomy for the treatment of cancer has been widely adopted in the United States. Dis Colon Rectum 2012;55:501-8. [Crossref] [PubMed]
- van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015;314:1356-63. [Crossref] [PubMed]
- Fleshman J, Branda ME, Sargent DJ, et al. Disease-free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer: Follow-up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann Surg 2019;269:589-95. [Crossref] [PubMed]
- Damle A, Damle RN, Flahive JM, et al. Diffusion of technology: Trends in robotic-assisted colorectal surgery. Am J Surg 2017;214:820-4. [Crossref] [PubMed]
- Bhama AR, Obias V, Welch KB, et al. A comparison of laparoscopic and robotic colorectal surgery outcomes using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Surg Endosc 2016;30:1576-84. [Crossref] [PubMed]
- Mirkin KA, Kulaylat AS, Hollenbeak CS, et al. Robotic versus laparoscopic colectomy for stage I-III colon cancer: oncologic and long-term survival outcomes. Surg Endosc 2018;32:2894-901. [Crossref] [PubMed]
- Cleary RK, Mullard AJ, Ferraro J, et al. The cost of conversion in robotic and laparoscopic colorectal surgery. Surg Endosc 2018;32:1515-24. [Crossref] [PubMed]
- Spinoglio G, Bianchi PP, Marano A, et al. Robotic Versus Laparoscopic Right Colectomy with Complete Mesocolic Excision for the Treatment of Colon Cancer: Perioperative Outcomes and 5-Year Survival in a Consecutive Series of 202 Patients. Ann Surg Oncol 2018;25:3580-6. Erratum in: Ann Surg Oncol 2019;26:884. [Crossref] [PubMed]
- Yozgatli TK, Aytac E, Ozben V, et al. Robotic Complete Mesocolic Excision Versus Conventional Laparoscopic Hemicolectomy for Right-Sided Colon Cancer. J Laparoendosc Adv Surg Tech A 2019;29:671-6. [Crossref] [PubMed]
- Yang Y, Malakorn S, Zafar SN, et al. Superior Mesenteric Vein-First Approach to Robotic Complete Mesocolic Excision for Right Colectomy: Technique and Preliminary Outcomes. Dis Colon Rectum 2019;62:894-7. [Crossref] [PubMed]
- Bae SU, Yang SY, Min BS. Totally robotic modified complete mesocolic excision and central vascular ligation for right-sided colon cancer: technical feasibility and mid-term oncologic outcomes. Int J Colorectal Dis 2019;34:471-9. [Crossref] [PubMed]
- Park JS, Kang H, Park SY, et al. Long-term oncologic after robotic versus laparoscopic right colectomy: a prospective randomized study. Surg Endosc 2019;33:2975-81. [Crossref] [PubMed]
- Ramachandra C, Sugoor P, Karjol U, et al. Robotic Complete Mesocolic Excision with Central Vascular Ligation for Right Colon Cancer: Surgical Technique and Short-term Outcomes. Indian J Surg Oncol 2020;11:674-83. [Crossref] [PubMed]
- Ceccarelli G, Costa G, Ferraro V, et al. Robotic or three-dimensional (3D) laparoscopy for right colectomy with complete mesocolic excision (CME) and intracorporeal anastomosis? A propensity score-matching study comparison. Surg Endosc 2021;35:2039-48. [Crossref] [PubMed]
- Siddiqi N, Stefan S, Jootun R, et al. Robotic Complete Mesocolic Excision (CME) is a safe and feasible option for right colonic cancers: short and midterm results from a single-centre experience. Surg Endosc 2021;35:6873-81. [Crossref] [PubMed]
- Bianchi PP, Salaj A, Giuliani G, et al. Feasibility of robotic right colectomy with complete mesocolic excision and intracorporeal anastomosis: short-term outcomes of 161 consecutive patients. Updates Surg 2021;73:1065-72. [Crossref] [PubMed]
- Khan JS, Ahmad A, Odermatt M, et al. Robotic complete mesocolic excision with central vascular ligation for right colonic tumours - a propensity score-matching study comparing with standard laparoscopy. BJS Open 2021;5:zrab016.
- Widmar M, Keskin M, Strombom P, et al. Lymph node yield in right colectomy for cancer: a comparison of open, laparoscopic and robotic approaches. Colorectal Dis 2017;19:888-94. [Crossref] [PubMed]
- Yuval JB, Thompson HM, Verheij FS, et al. Comparison of Robotic, Laparoscopic, and Open Resections of Nonmetastatic Colon Cancer. Dis Colon Rectum 2023;66:1347-58. [Crossref] [PubMed]
- Cuk P, Jawhara M, Al-Najami I, et al. Robot-assisted versus laparoscopic short- and long-term outcomes in complete mesocolic excision for right-sided colonic cancer: a systematic review and meta-analysis. Tech Coloproctol 2023;27:171-81. [Crossref] [PubMed]
- Zhang H, Sun N, Fu Y, et al. Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: updated meta-analysis of randomized controlled trials. BJS Open 2021;5:zrab133.
- Cleary RK, Silviera M, Reidy TJ, et al. Intracorporeal and extracorporeal anastomosis for robotic-assisted and laparoscopic right colectomy: short-term outcomes of a multi-center prospective trial. Surg Endosc 2022;36:4349-58. [Crossref] [PubMed]
- Widmar M, Aggarwal P, Keskin M, et al. Intracorporeal Anastomoses in Minimally Invasive Right Colectomies Are Associated With Fewer Incisional Hernias and Shorter Length of Stay. Dis Colon Rectum 2020;63:685-92. [Crossref] [PubMed]
- Trastulli S, Coratti A, Guarino S, et al. Robotic right colectomy with intracorporeal anastomosis compared with laparoscopic right colectomy with extracorporeal and intracorporeal anastomosis: a retrospective multicentre study. Surg Endosc 2015;29:1512-21. [Crossref] [PubMed]
- Sorgato N, Mammano E, Contardo T, et al. Right colectomy with intracorporeal anastomosis for cancer: a prospective comparison between robotics and laparoscopy. J Robot Surg 2022;16:655-63. [Crossref] [PubMed]
- Tekkis PP, Senagore AJ, Delaney CP, et al. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 2005;242:83-91. [Crossref] [PubMed]
- Parisi A, Scrucca L, Desiderio J, et al. Robotic right hemicolectomy: Analysis of 108 consecutive procedures and multidimensional assessment of the learning curve. Surg Oncol 2017;26:28-36. [Crossref] [PubMed]
- de'Angelis N, Lizzi V, Azoulay D, et al. Robotic Versus Laparoscopic Right Colectomy for Colon Cancer: Analysis of the Initial Simultaneous Learning Curve of a Surgical Fellow. J Laparoendosc Adv Surg Tech A 2016;26:882-92. [Crossref] [PubMed]
- Justiniano CF, Becerra AZ, Xu Z, et al. A Population-Based Study of 90-Day Hospital Cost and Utilization Associated With Robotic Surgery in Colon and Rectal Cancer. J Surg Res 2020;245:136-44. [Crossref] [PubMed]
- Barbash GI, Glied SA. New technology and health care costs--the case of robot-assisted surgery. N Engl J Med 2010;363:701-4. [Crossref] [PubMed]
- Keller DS, Senagore AJ, Lawrence JK, et al. Comparative effectiveness of laparoscopic versus robot-assisted colorectal resection. Surg Endosc 2014;28:212-21. [Crossref] [PubMed]
- Day EK, Galbraith NJ, Ward HJT, et al. Volume-outcome relationship in intra-abdominal robotic-assisted surgery: a systematic review. J Robot Surg 2023;17:811-26. [Crossref] [PubMed]
Cite this article as: Quezada-Diaz FF, Smith JJ. Colon cancer: is the robot a better option? Ann Laparosc Endosc Surg 2023;8:35.


