Endoscopic submucosal dissection of neoplastic lesions of the colon: clinical application and techniques
Introduction
Background
Endoscopic submucosal dissection (ESD), a widely established endoscopic modality that was first developed in Japan, has been increasingly performed in Asia, Europe, and the United States (1,2). ESD has become a mature modality that can be safely performed throughout the gastrointestinal tract, including the esophagus, stomach, duodenum, and colon (3). ESD allows en bloc removal of large superficial lesions, reduces the risk of local recurrence, and allows precise pathological evaluations (3). When comparing ESD to endoscopic mucosal resection (EMR), ESD yields higher en bloc resection rates (4) and lower local recurrence rates (0–2.7% vs. 6.8–23.5%) (5-10). Colorectal ESD is most commonly performed in the US because of the established colorectal cancer (CRC) screening program and the higher prevalence of colorectal lesions, as compared to that of upper gastrointestinal lesions (11).
Rationale and knowledge gap
Compared to surgical removal, as an organ-sparing procedure, ESD is better tolerated by patients, reducing complication rates, lengths of hospital stays, and costs while achieving similar outcomes for complete resection and recurrence rates (12,13). However, ESD is technically more challenging and time-consuming than EMR and is not suitable for advanced lesions with lymph node metastasis (14,15). Therefore, careful patient selection, knowledge of appropriate indications, and a deep understanding of the technical aspects of ESD are crucial to safely and effectively perform ESD procedures.
Objective
This article discusses the indications for colorectal ESD, describes the technical aspects and variations, and outlines the clinical outcomes.
Colorectal ESD
Indications
Colorectal ESD is most appropriate for large lesions (≥20 mm) with a risk of submucosal invasion and lesions with significant fibrosis due to prior resection attempts or tattooing (Figure 1) (2,16). For lower-risk lesions [e.g., homogeneously granular lesions with a Japan narrow-band imaging (NBI) Expert Team (JNET) classification of 2A] (17), traditional EMR may be more appropriate and can lead to lower adverse event rates and reduced procedural times; however, patients should be informed of the increased risk of local recurrence due to a piecemeal resection. These patients should undergo colonoscopy at short intervals (3–6 months) for surveillance and additional treatment for local recurrence (14,18,19).

For high-risk lesions [e.g., lesions with a depressed component (Paris classification of 0–IIc), non-granular lesions, granular lesions with a large nodule (>2 cm), and those with JNET classification of 2B], and lesions with submucosal fibrosis (Figure 1) (10,16), en bloc resection with ESD is highly recommended. Even though these lesions are considered to have a high risk, the risk of covert invasive cancer is estimated to be 3.7–15.3% (16). Thus, most high-risk lesions can be curatively treated using ESD. Moreover, even for possible stage T1b lesions, ESD can be used as a staging procedure to accurately evaluate the invasion depth (stage T1a or T1b) and other risk factors for lymph node metastasis (tumor differentiation, invasion depth, lymphovascular invasion, and tumor budding).
Lesions with clear evidence of a T1b stage (invasion depth >1,000 µm) or greater, such as deep ulcerations, an obstructing mass, and a JNET classification of 3, should be directly referred for colorectal surgery and surgical resection (18,20) because of the risk of lymph node metastasis.
Description of the ESD technique
Conventional ESD is performed in multiple sequential steps (3) (Figure 2). First, the lesion borders are visualized by white-light endoscopy, image-enhanced endoscopy [NBI or blue-light imaging (BLI)], or chromoendoscopy. Then, if the boundaries of the lesion are unclear, thermal dots can be applied using the tip of the ESD knives to mark the periphery, ideally 3–5 mm outside the lesion borders. Following submucosal injection, a mucosal incision is performed circumferentially or partially depending on the strategy in ESD (see Variations of the ESD technique) using a specialized ESD knife. Table 1 shows a list of commercially available devices in the US for colorectal ESD (21-28).
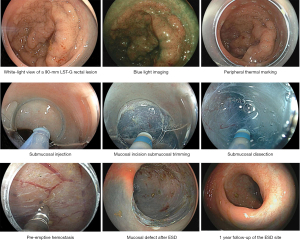
Table 1
| Images | Name | Company | Tip type | Injection capability | Other features |
|---|---|---|---|---|---|
 |
DualKnife/DualKnife-J | Olympus America | Needle | DualKnife-J only | Injection via the catheter |
 |
ORISE ProKnife | Boston Scientific | Needle | Yes | Injection via the needle |
 |
HybridKnife | ERBE USA | Needle | Yes (regulated pressure water jet) | A dedicated ERBEJET 2 pump cartridge is required for injection |
 |
FlushKnife BT-S/N-S | Fujifilm | Ball tip vs. Needle | Yes | Injection via the catheter |
 |
HookKnife/HookKnife-J | Olympus America | Needle | HookKnife-J only | Hook can be helpful in cases with severe fibrosis or poor maneuverability |
 |
Speedboat Inject | Creo Medical | Needle | Yes | Bipolar energy for dissection and microwave energy for coagulation |
 |
Itknife Nano | Olympus America | Insulated | No | Tip designed to minimize the risk of muscle injury |
 |
SB Knife | Olympus America | Scissor | No | Allows for hemostasis and dissection |
 |
Clutch Cutter | Fujifilm | Scissor | No | Allows for hemostasis and dissection |
 |
ProdiGI Multi-functional ESD Knife | Medtronic | Ball tip and insulated (2-in-1) | No | Knife tip can be changed to help maneuver in smaller spaces or insulate against deep mucosa |
All images were provided with permission from their respective companies, including Olympus America, Fujifilm, Creo Medical, ERBE USA (©Erbe Elektromedizin GmbH), Medtronic, and Boston Scientific (©2023 Boston Scientific Corporation or its affiliates). All rights reserved. ESD, endoscopic submucosal dissection.
For submucosal dissection, the endoscope tip with an attachment cap is inserted into the submucosal space immediately after performing “submucosal trimming”, to create a mucosal flap. The submucosal tissue is then slowly dissected parallel to and above the muscularis propria. The submucosal dissection continues until the lesion is completely removed from the muscularis propria. The deeper parts of the submucosal layer can be preserved if the suspicion of deep submucosal invasion is low. This step is often performed in parallel with submucosal injection to lift the lesion if the ESD knife allows simultaneous injections (Table 1), separating the mucosa from the muscular layer to reduce the risk of thermal muscle injury and perforation (29). Submucosal injection agents range from simple solvent solutions (e.g., normal saline and dextrose water) to more complex and often proprietary osmotic agent solutions, the latter of which often allows better and long-lasting submucosal elevation (29-34) (Table 2). At our institution, we favor hydroxyethyl starch because of the reasonable cost and excellent submucosal elevation properties. However, the choice of agent can vary among institutions, based on the provider preference, cost, and product availability.
Table 2
| Solution | Company | Submucosal elevation | Cost | Additional downside |
|---|---|---|---|---|
| Normal saline | N/A | Poor | Inexpensive | None |
| Dextrose water | N/A | Moderate | Inexpensive | High dextrose concentrations may result in tissue damage (33) |
| Hydroxyethyl starch (HES) | N/A | Excellent | Reasonable | None |
| Hydroxypropyl methylcellulose (HPMC) | N/A | Moderate | Reasonable | Can rarely cause antigen reactions (34) |
| Hyaluronic acid (HA) | N/A | Excellent | Expensive | May trigger growth of any remaining tumor cells (32) |
| Eleview | Cosmo Pharmaceuticals | Excellent | Expensive | None |
| Blueboost | Microtech Endoscopy | Excellent | Expensive | None |
| Everlift | Laborie | Excellent | Expensive | None |
| Endoclot | Olympus America | Excellent | Expensive | None |
ESD, endoscopic submucosal dissection; N/A, not applicable.
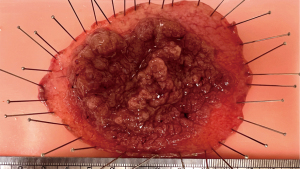
Once ESD is completed, careful inspection of the mucosal defect is performed to ensure that no muscle injury is visible. The specimens are retrieved using a large oval retrieval net. Coagulation with a soft coagulation current is then performed using hemostatic forceps on any exposed, non-bleeding, visible vessels or areas of bleeding at the endoscopist’s discretion (35). The resected specimen is then secured onto a flat cork or rubber board with pins to ensure no edge curling, allowing the pathologist to assess the specimen’s size, vertical and lateral margins, invasion depth, tumor budding, and presence of lymphovascular invasion (Figure 3).
In North America and Europe, colorectal ESDs are mostly performed under monitored anesthesia care or general anesthesia. In Asia, conscious sedation is more prevalent, owing to the limited access to anesthesia support. However, patient restlessness and discomfort may be more challenging to manage, especially for longer cases, and propofol may be necessary for cases requiring longer procedural times (36).
Variations of the ESD technique
In general, colorectal ESD is more technically challenging than esophageal and gastric ESD because of numerous factors, such as the anatomical complexity, thinner submucosal layer, and scope instability (37). Lesions at the ileocecal valve, flexure areas, and sigmoid colon are particularly challenging. Although some technical challenges can be overcome with standard endoscopic maneuvers (e.g., loop reduction, changing the dependent field, switching to a pediatric colonoscope or upper endoscope for better maneuverability and retroflexion), many cases can remain highly technically difficult. Thus, the ESD technique has been modified over the years to mitigate some of the difficulties in colorectal ESD.
One modification is the pocket-creation method, in which, instead of completing a circumferential mucosal incision, only a short horizontal mucosal incision is made before proceeding with submucosal dissection. Once the endoscope is in the submucosal space, most of the submucosal tissue underneath the lesion is dissected by creating a large “pocket” (38,39). Although the submucosal entry site where the submucosal dissection occurs is small, the endoscope is better stabilized in this pocket. Therefore, submucosal dissection can be less affected by fluctuations secondary to peristaltic movements. One systematic review and meta-analysis found that the pocket-creation method may lead to a higher en bloc and R0 resection rates, with a pooled absolute risk difference of 5% for the en bloc resection rate and 14% for the R0 resection rate (40). However, this difference was not observed in a randomized controlled trial (RCT), as en bloc and R0 resection rates in both groups were 100% (41). In addition, there were notable advantages to the saline-pocket ESD method described in this RCT, including faster median dissection speed (average of 20.1 for saline-pocket ESD vs. 16.3 mm2/min for standard ESD, P<0.001) and faster median procedure time (average of 29.5 vs. 41 minutes respectively, P<0.001) (41). No perforations were noted among patients.
Another technique that aims to address the limitations of ESD is the underwater (or water pressure) ESD technique (42-45). Right after submucosal injection, instead of proceeding straight to cutting, the colon lumen is filled with a saline solution with the patient positioned so that the lesion can be easily submerged. The saline solution creates a “floating” effect in that dissected mucosal and submucosal tissue, when submerged, tends to float away from the mucosal layer against gravity—this creates natural traction during submucosal dissection and allows for easy identification of underlying submucosal and muscular layers (42). This technique can be particularly useful for cases in which the lesion has severe submucosal fibrosis. One retrospective study of 133 patients compared conventional ESD to underwater ESD for lesions with expected submucosal fibrosis and found underwater ESD had shorter procedure time (43.5 vs. 72 min, P=0.0041) with a similar safety profile, including perforation risk (45).
A third method to highlight is the general category of traction techniques. As submucosal dissection advances further into the center of the lesion, previously dissected areas are more affected by gravity and peristaltic movements, which can interfere with the field of vision during dissection (46). One major advantage of using a traction device is the stability of the mucosal flap and direct visualization of the submucosal dissection plane, making ESD technically less challenging and theoretically safer to perform (47). Mitsuyoshi et al. conducted a study examining the ESD trainees’ learning curve using a simple traction device consisting of two clips linked by a nylon suture loop, with one clip attached to an edge of the semi-dissected lesion and another clip fixed to the opposite wall of the colon. The study demonstrated that trainees using the traction device had a higher self-completion rate (74% vs. 59% without a traction device), en bloc resection rate (100% vs. 90%), and R0 resection rate (96% vs. 83%) (48). Several traction devices are commercially available in the US, such as the SureTrac elastic traction device (MicroTech Endoscopy USA, Ann Arbor, MI, USA), ProdiGI Traction Wire (Medtronic, Minneapolis, MN, USA), and TracMotion (FujiFilm, Tokyo, Japan). Yang et al. conducted a prospective ex vivo multicenter study with the Tracmotion device and demonstrated that trainees using the device had significantly higher dissection speeds (43.32±22.61 vs. 24.19±15.86 mm2/min; P=0.042) and reduced mental and physical loads based on a quantitative scoring system, though the study participant size was too small to detect differences in en bloc and R0 resection rates (49).
Lastly, other modified ESD techniques include hybrid ESD and precutting EMR techniques (3). In precutting EMR, a peripheral mucosal incision is first performed. The lesion isolated from the surrounding normal mucosa is removed en bloc using a hot snare (50). Similar to conventional ESD, circumferential mucosal incision and submucosal dissection are performed during hybrid ESD. However, only partial submucosal dissection is performed. Once the remaining submucosal area is sufficiently small (<15 mm in size), the remaining portion of the procedure is completed with snare resection. The main advantage of precutting EMR and the hybrid ESD technique is a reduced procedural time and lower technical difficulty, given that the most technically challenging part of ESD often lies in the final portion of the submucosal dissection, considering the reduced lesion stability. Hybrid ESD can be particularly helpful for endoscopists earlier on in their ESD training and in some cases for more experienced endoscopists snare resection in the last stage may also be less risky or more positionally favored (51-53). However, the main concern for precutting EMR and the hybrid ESD technique is the risk of a lower en bloc resection rate with subsequent use of EMR (51), with a decreased odds ratio of 0.31 (P<0.001) in achieving an en bloc resection rate reported in a systematic review and meta-analysis (54).
Overall, these modified ESD techniques have advantages and disadvantages, and the choice may depend on the endoscopist’s experience and institutional availability of ancillary devices.
Outcomes
ESD is a safe and well-tolerated alternative to surgery and has been validated across numerous multicenter prospective cohort studies across Asia, Europe, and North America (11,55,56). Fleischmann et al. demonstrated that given the high technical difficulty of the procedures, outcomes are better at high-volume centers with significantly higher en bloc resection rates (96% for high-volume vs. 86% for low-volume centers), higher R0 resection rates (88% for high-volume vs. 69% for low-volume centers), and lower adverse event rates (3% at high-volume and 13% and 11% at middle- and low-volume centers, respectively) (55). According to a recent US multicenter study, colorectal ESD yielded a significantly lower en bloc resection rate (85.8% in the colon and 88.8% in the rectum) than those for esophageal and gastric ESD (96.7% in the esophagus and 98.0% in the stomach) (11). The US-based study also revealed a lower en bloc resection rate than that reported in a Japanese multicenter study (97.0%) (38). This is potentially due to the increased difficulty of colorectal ESD in the US compared with that in Japan.
According to a study by Ge et al. analyzing the clinical outcomes of their experience in colorectal ESD in the US, 16.9% of colorectal lesions had tattoos underneath the lesion, and 29.9% had previous EMR attempts (57). These findings resulted in a significantly high number of fibrotic lesions (F1: 45.5%, F2: 23.4%), and on multivariate analysis, the presence of a tattoo predicted the failure to achieve curative resection [odds ratio (OR): 0.13; 95% confidence interval (CI): 0.02–0.98; P=0.048]. In addition, the presence of a tattoo (OR: 9.38; 95% CI: 1.05–83.83; P=0.045) and prior EMR attempts (OR: 7.13; 95% CI: 1.76–28.90; P=0.006) could be used as factors predicting an ESD procedure time ≥90 min. In contrast, in Japan, these maneuvers are strictly avoided to prevent negative ESD outcomes, such as those mentioned previously. Therefore, tattooing underneath a lesion should always be avoided. EMR should not be attempted if the initial endoscopist does not confidently believe that complete resection is feasible on the first attempt. Lastly, ESD cases in North America and Europe may be more difficult than those in Japan as a result of higher prevalence of obesity. Central obesity may correlate with poor scope maneuverability and increased density of submucosal fatty tissue, which can cause submucosal dissection to become technically more challenging (58,59). From an anesthesia perspective, patients with obesity in addition pose additional issues of sedation difficulties and increased respiratory variations which can make the ESD case even more challenging to perform, requiring general anesthesia for most cases (59).
Regarding adverse events in colorectal ESD, in North America, the respective delayed bleeding and perforation rates are 1.9% and 4.7% in the colon and 2.7% and 2.7% in the rectum (11). These numbers appear to be equivalent to the data from Japan, where perforation and delayed bleeding rates are 2.0–10.7% and 1.5–2.8%, respectively (60-66), with one study out of 1,883 patients separating intra- and postprocedural perforation rate at 2.6% and 0.6% respectively as well as 0.5% patients requiring emergency surgery post procedure (67).
Interpretation of pathological findings
En bloc ESD specimens provide several essential pathological findings to determine the endoscopic treatment’s curative ability (e.g., vertical and horizontal margins, tumor differentiation, invasion depth, lymphovascular invasion, and tumor budding). These factors are directly associated with the risk of lymph node involvement, and their correct interpretation is important to determine if patients require additional treatment or if endoscopic surveillance is sufficient. The ESD specimens should be appropriately processed for accurate histopathological diagnosis as described in the Japanese ESD guideline (68).
Guidelines from the European and Japanese Societies of Gastrointestinal Endoscopy (68,69) define the curative resection of ESD as “en bloc R0 resection of the lesion with up to superficial submucosal invasion (T1a) that is well to moderately differentiated, with no lymphovascular invasion and no grade 2 or 3 budding”. Proper communication with pathologists specializing in gastrointestinal pathology is crucial to accurately determine the curability of endoscopic resection based on these findings. Because there are still no ESD guidelines in the US, even if the pathological findings meet the criteria for curative resection, T1a cases with no other risk factors should be discussed by a multidisciplinary tumor board. On the other hand, non-curative resection cases should be immediately referred for surgery to determine the patient’s surgical candidacy.
Conclusions
ESD yields the advantage of avoiding surgery and harbors higher en bloc and curative rates than those in EMR but may lead to higher adverse event rates as a tradeoff. However, the latter risk may be mitigated at high-volume centers with more experienced endoscopists. Therefore, a solid understanding of the indications and outcomes for ESD is crucial for both general and advanced endoscopists to allow for the best and safest outcomes for patients with advanced colorectal lesions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Terry L. Jue) for the series “A U.S. Perspective on Endoscopic Resection of Neoplastic Lesions of the Gastrointestinal Tract” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-12/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-12/coif). The series “A U.S. Perspective on Endoscopic Resection of Neoplastic Lesions of the Gastrointestinal Tract” was commissioned by the editorial office without any funding or sponsorship. HA serves as a consultant for Olympus America, Boston Scientific, and Fujifilm Medical Systems and received a research support from Boston Scientific. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The images demonstrated in
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tanaka S, Oka S, Kaneko I, et al. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc 2007;66:100-7. [Crossref] [PubMed]
- Draganov PV, Wang AY, Othman MO, et al. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16-25.e1. [Crossref] [PubMed]
- Ge PS, Aihara H. Advanced Endoscopic Resection Techniques: Endoscopic Submucosal Dissection and Endoscopic Full-Thickness Resection. Dig Dis Sci 2022;67:1521-38. [Crossref] [PubMed]
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015;81:583-95. [Crossref] [PubMed]
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010;24:343-52. [Crossref] [PubMed]
- Hotta K, Saito Y, Matsuda T, et al. Local recurrence and surveillance after endoscopic resection of large colorectal tumors. Dig Endosc 2010;22:S63-8. [Crossref] [PubMed]
- Sakamoto T, Matsuda T, Otake Y, et al. Predictive factors of local recurrence after endoscopic piecemeal mucosal resection. J Gastroenterol 2012;47:635-40. [Crossref] [PubMed]
- Oka S, Tanaka S, Saito Y, et al. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015;110:697-707. [Crossref] [PubMed]
- Hotta K, Fujii T, Saito Y, et al. Local recurrence after endoscopic resection of colorectal tumors. Int J Colorectal Dis 2009;24:225-30. [Crossref] [PubMed]
- Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 2011;140:1909-18. [Crossref] [PubMed]
- Draganov PV, Aihara H, Karasik MS, et al. Endoscopic Submucosal Dissection in North America: A Large Prospective Multicenter Study. Gastroenterology 2021;160:2317-2327.e2. [Crossref] [PubMed]
- Gu L, Khadaroo PA, Chen L, et al. Comparison of Long-Term Outcomes of Endoscopic Submucosal Dissection and Surgery for Early Gastric Cancer: a Systematic Review and Meta-analysis. J Gastrointest Surg 2019;23:1493-501. [Crossref] [PubMed]
- Kiriyama S, Saito Y, Yamamoto S, et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy 2012;44:1024-30. [Crossref] [PubMed]
- De Ceglie A, Hassan C, Mangiavillano B, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol 2016;104:138-55. [Crossref] [PubMed]
- Gangireddy VGR, Coleman T, Kanneganti P, et al. Polypectomy versus surgery in early colon cancer: size and location of colon cancer affect long-term survival. Int J Colorectal Dis 2018;33:1349-57. [Crossref] [PubMed]
- Burgess NG, Hourigan LF, Zanati SA, et al. Risk Stratification for Covert Invasive Cancer Among Patients Referred for Colonic Endoscopic Mucosal Resection: A Large Multicenter Cohort. Gastroenterology 2017;153:732-742.e1. [Crossref] [PubMed]
- Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc 2016;28:526-33. [Crossref] [PubMed]
- Arezzo A, Passera R, Marchese N, et al. Systematic review and meta-analysis of endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal lesions. United European Gastroenterol J 2016;4:18-29. [Crossref] [PubMed]
- Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic Removal of Colorectal Lesions-Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020;158:1095-129. [Crossref] [PubMed]
- Gorgun E, Benlice C, Abbas MA, et al. Experience in colon sparing surgery in North America: advanced endoscopic approaches for complex colorectal lesions. Surg Endosc 2018;32:3114-21. [Crossref] [PubMed]
- Oka S, Tanaka S, Takata S, et al. Usefulness and safety of SB knife Jr in endoscopic submucosal dissection for colorectal tumors. Dig Endosc 2012;24:90-5. [Crossref] [PubMed]
- Yoshii S, Yamano HO, Nakase H. Novel injectable needle-type knife, ProKnife for colorectal endoscopic submucosal dissection. Dig Endosc 2021;33:e137-9. [Crossref] [PubMed]
- Jacques J, Legros R, Charissoux A, et al. A combination of pocket, double-clip countertraction, and isolated HybridKnife as a quick and safe strategy for colonic endoscopic submucosal dissection. Endoscopy 2017;49:E134-5. [Crossref] [PubMed]
- Takeuchi Y, Uedo N, Ishihara R, et al. Efficacy of an endo-knife with a water-jet function (Flushknife) for endoscopic submucosal dissection of superficial colorectal neoplasms. Am J Gastroenterol 2010;105:314-22. [Crossref] [PubMed]
- McCarty TR, Aihara H. Endoscopic submucosal tunneling dissection: use of a novel bipolar radiofrequency and microwave-powered device for colorectal endoscopic submucosal dissection. VideoGIE 2020;5:335-8. [Crossref] [PubMed]
- Imai K, Hotta K, Yamaguchi Y, et al. Endoscopic submucosal dissection for large colorectal neoplasms. Dig Endosc 2017;29:53-7. [Crossref] [PubMed]
- Akahoshi K, Akahane H, Motomura Y, et al. A new approach: endoscopic submucosal dissection using the Clutch Cutter® for early stage digestive tract tumors. Digestion 2012;85:80-4. [Crossref] [PubMed]
- Inoue H, Santi EG, Onimaru M, et al. Submucosal endoscopy: from ESD to POEM and beyond. Gastrointest Endosc Clin N Am 2014;24:257-64. [Crossref] [PubMed]
- Castro R, Libânio D, Pita I, et al. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol 2019;25:777-88. [Crossref] [PubMed]
- Najarian RM. EverLift® Submucosal Lifting Agent results in no foreign body artifacts post endoscopic mucosal resection and endoscopic submucosal dissection.
- Dai MS, Hu KW, Wu W, et al. EndoClot(®)SIS Polysaccharide Injection as a Submucosal Fluid Cushion for Endoscopic Mucosal Therapies: Results of Ex Vivo and In Vivo Studies. Dig Dis Sci 2019;64:2955-64. [Crossref] [PubMed]
- Matsui Y, Inomata M, Izumi K, et al. Hyaluronic acid stimulates tumor-cell proliferation at wound sites. Gastrointest Endosc 2004;60:539-43. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kashimura K, et al. Tissue damage of different submucosal injection solutions for EMR. Gastrointest Endosc 2005;62:933-42. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kashimura K, et al. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy 2004;36:579-83. [Crossref] [PubMed]
- Bahin FF, Naidoo M, Williams SJ, et al. Prophylactic endoscopic coagulation to prevent bleeding after wide-field endoscopic mucosal resection of large sessile colon polyps. Clin Gastroenterol Hepatol 2015;13:724-30.e1-2.
- Yoshida N, Yagi N, Naito Y, et al. Safe procedure in endoscopic submucosal dissection for colorectal tumors focused on preventing complications. World J Gastroenterol 2010;16:1688-95. [Crossref] [PubMed]
- Uraoka T, Parra-Blanco A, Yahagi N. Colorectal endoscopic submucosal dissection: is it suitable in western countries? J Gastroenterol Hepatol 2013;28:406-14. [Crossref] [PubMed]
- Takezawa T, Hayashi Y, Shinozaki S, et al. The pocket-creation method facilitates colonic endoscopic submucosal dissection (with video). Gastrointest Endosc 2019;89:1045-53. [Crossref] [PubMed]
- Hayashi Y, Miura Y, Yamamoto H. Pocket-creation method for the safe, reliable, and efficient endoscopic submucosal dissection of colorectal lateral spreading tumors. Dig Endosc 2015;27:534-5. [Crossref] [PubMed]
- Gong J, Chen T, Tan Y, et al. Pocket-creation method improves efficacy of colorectal endoscopic submucosal dissection: a system review and meta-analysis. Eur J Gastroenterol Hepatol 2021;33:1241-6. [Crossref] [PubMed]
- Harada H, Nakahara R, Murakami D, et al. Saline-pocket endoscopic submucosal dissection for superficial colorectal neoplasms: a randomized controlled trial (with video). Gastrointest Endosc 2019;90:278-87. [Crossref] [PubMed]
- Yoshii S, Akasaka T, Hayashi Y, et al. "Underwater" endoscopic submucosal dissection: a novel method for resection in saline with a bipolar needle knife for colorectal epithelial neoplasia. Surg Endosc 2018;32:5031-6. [Crossref] [PubMed]
- Nagata M. Usefulness of underwater endoscopic submucosal dissection in saline solution with a monopolar knife for colorectal tumors (with videos). Gastrointest Endosc 2018;87:1345-53. [Crossref] [PubMed]
- Ramos-Zabala F, Beg S, García-Mayor M, et al. Novel approach to endoscopic submucosal dissection of a cecal lesion with nonlifting sign by submucosal fatty tissue with use of selective-regulation high-pressure water-jet method and immersion in saline solution. VideoGIE 2020;5:116-9. [Crossref] [PubMed]
- Ozeki Y, Hirasawa K, Ikeda R, et al. Safety and efficacy of water pressure endoscopic submucosal dissection for colorectal tumors with submucosal fibrosis (with video). Gastrointest Endosc 2021;94:607-617.e2. [Crossref] [PubMed]
- Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc 2012;45:375-8. [Crossref] [PubMed]
- Ide D, Saito S, Ohya TR, et al. Colorectal endoscopic submucosal dissection can be efficiently performed by a trainee with use of a simple traction device and expert supervision. Endosc Int Open 2019;7:E824-32. [Crossref] [PubMed]
- Mitsuyoshi Y, Ide D, Ohya TR, et al. Training program using a traction device improves trainees' learning curve of colorectal endoscopic submucosal dissection. Surg Endosc 2022;36:4462-9. [Crossref] [PubMed]
- Yang D, Aihara H, Hasan MK, et al. Novel single-operator through-the-scope traction device for endoscopic submucosal dissection: Outcomes of a multicenter randomized pilot ex-vivo study in trainees with limited endoscopic submucosal dissection experience (with video). DEN Open 2023;3:e174. [Crossref] [PubMed]
- Yoshida N, Inoue K, Dohi O, et al. Precutting EMR with full or partial circumferential incision with a snare tip for the en bloc resection of difficult colorectal lesions. VideoGIE 2018;3:378-80. [Crossref] [PubMed]
- Okamoto K, Muguruma N, Kagemoto K, et al. Efficacy of hybrid endoscopic submucosal dissection (ESD) as a rescue treatment in difficult colorectal ESD cases. Dig Endosc 2017;29:45-52. [Crossref] [PubMed]
- Pérez-Cuadrado-Robles E, Snauwaert C, Moreels TG, et al. Risk factors for conversion to snare resection during colorectal endoscopic submucosal dissection in an expert Western center. Endoscopy 2019;51:152-60. [Crossref] [PubMed]
- Steinbrück I, Faiss S, Dumoulin FL, et al. Learning curve of endoscopic submucosal dissection (ESD) with prevalence-based indication in unsupervised Western settings: a retrospective multicenter analysis. Surg Endosc 2023;37:2574-86. [Crossref] [PubMed]
- McCarty TR, Bazarbashi AN, Thompson CC, et al. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy 2021;53:1048-58. [Crossref] [PubMed]
- Fleischmann C, Probst A, Ebigbo A, et al. Endoscopic Submucosal Dissection in Europe: Results of 1000 Neoplastic Lesions From the German Endoscopic Submucosal Dissection Registry. Gastroenterology 2021;161:1168-78. [Crossref] [PubMed]
- Ohata K, Kobayashi N, Sakai E, et al. Long-term Outcomes After Endoscopic Submucosal Dissection for Large Colorectal Epithelial Neoplasms: A Prospective, Multicenter, Cohort Trial From Japan. Gastroenterology 2022;163:1423-1434.e2. [Crossref] [PubMed]
- Ge PS, Jirapinyo P, Ohya TR, et al. Predicting outcomes in colorectal endoscopic submucosal dissection: a United States experience. Surg Endosc 2019;33:4016-25. [Crossref] [PubMed]
- Ramos-Zabala F, Parra-Blanco A, Beg S, et al. The impact of submucosal fatty tissue during colon endoscopic submucosal dissection in a western center. Eur J Gastroenterol Hepatol 2021;33:1063-70. [Crossref] [PubMed]
- Tachikawa J, Chiba H, Okada N, et al. Impact of obesity in colorectal endoscopic submucosal dissection: single-center retrospective cohort study. BMC Gastroenterol 2021;21:74. [Crossref] [PubMed]
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013;27:3262-70. [Crossref] [PubMed]
- Oka S, Tanaka S, Kanao H, et al. Current status in the occurrence of postoperative bleeding, perforation and residual/local recurrence during colonoscopic treatment in Japan. Dig Endosc 2010;22:376-80. [Crossref] [PubMed]
- Fujishiro M, Uemura N, Tanaka S. Report on analysis of colorectal ESD data. ‘JGES prospective multicenter cohort study on effectiveness and safety of colorectal ESD conducted as Advanced Medical Treatment: A brief outline and future plan.’ Gastroenterol Endosc 2013;55:1331.
- Wada Y, Kudo SE, Tanaka S, et al. Predictive factors for complications in endoscopic resection of large colorectal lesions: a multicenter prospective study. Surg Endosc 2015;29:1216-22. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kakushima N, et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol 2007;5:678-83; quiz 645. [Crossref] [PubMed]
- Isomoto H, Nishiyama H, Yamaguchi N, et al. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 2009;41:679-83. [Crossref] [PubMed]
- Watabe H, Yamaji Y, Okamoto M, et al. Risk assessment for delayed hemorrhagic complication of colonic polypectomy: polyp-related factors and patient-related factors. Gastrointest Endosc 2006;64:73-8. [Crossref] [PubMed]
- Kobayashi N, Takeuchi Y, Ohata K, et al. Outcomes of endoscopic submucosal dissection for colorectal neoplasms: Prospective, multicenter, cohort trial. Dig Endosc 2022;34:1042-51. [Crossref] [PubMed]
- Pimentel-Nunes P, Libânio D, Bastiaansen BAJ, et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy 2022;54:591-622. [Crossref] [PubMed]
- Tanaka S, Kashida H, Saito Y, et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2020;32:219-39. [Crossref] [PubMed]
Cite this article as: Wang TJ, Aihara H. Endoscopic submucosal dissection of neoplastic lesions of the colon: clinical application and techniques. Ann Laparosc Endosc Surg 2023;8:29.