
Kidney cancer: 3D laparoscopic partial nephrectomy—role in the current era of robotic surgery—a narrative review
Introduction
Minimally invasive surgeries have proven superior to open approaches in complex urological procedures such as partial nephrectomy (PN). Compared with open approaches, there was a significant reduction in intraoperative bleeding, postoperative complications, and length of hospitalization (LOH) with minimally invasive surgical procedures (1,2). Laparoscopic approach was the forerunner in the minimally invasive techniques, significantly improving the safety and efficacy of nephron-sparing surgery over the radical nephrectomy (RN) for renal cell carcinoma. It is the gold standard treatment modality for treating T1 renal tumours (3). Due to increased cardiovascular morbidity and a significant reduction in renal function following RN, PN cemented its role in the management of small renal masses as well as large tumours that are amenable to complete resection (4). With the advances made in minimally invasive surgery, the size of the primary renal mass is no longer considered a limiting factor for performing a successful PN (5-7).
The introduction of surgical robots has revolutionized the minimally invasive urological surgery field. Until recently, DaVinci surgical systems was the only commercially available surgical system that obtained Food and Drug Administration (FDA) approval in the United States in 2001 (8). However, many private companies have designed and developed surgical robots with similar potential (9,10). Since then, there has been a steady increase in the adoption of these surgical robots, especially in general surgery, gynaecology and urology. In a study conducted in 2020 in 73 hospitals, the proportion of robotic surgeries increased from 1.8% in 2012 to 15.1% in 2018, showing the enthusiasm to adopt emerging surgical modalities (11). However, due to the immense costs associated with surgical robots, their universal adoption in performing routine robotic partial nephrectomy (RPN) does not seem feasible in the near future.
On the other hand, significant advances were made to the existing laparoscopic systems that enable surgeons to perform LPN more efficiently. The introduction of three-dimensional (3D) visual systems, articulating instruments, intraoperative untrasonography, and firefly technology are some notable interventions successfully employed with varied success rates in performing laparoscopic renal surgeries (2,12,13). In a recent study, the ease of performing surgical tasks was found to be better with 3D laparoscopic vision than with two-dimensional (2D) imaging laparoscopy (14). Intracorporal suturing remains the Achilles heel with the laparoscopic approach compared to the robotic systems. Thus, the urological procedures involving intracorporal suturing, such as PN, nephroureterectomy with bladder cuff excision, and partial cystectomy, were generally considered difficult with a laparoscopic approach.
This review rigorously assesses how the integration of 3D laparoscopic vision influences oncological and perioperative outcomes in LPN. The absence of direct comparative studies between 3D-LPN and the established superior RPN accentuates the critical relevance of this review in contemporary minimally invasive renal cancer treatment. We present this article in accordance with the Narrative Review reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-23-38/rc).
Methods
This is a non-systemic literature review on the outcomes of 3D-LPN and RPN performed for renal masses. PubMed, Emabse, Clinicaltrials.gov, Clinical key and grey literature, including google scholar databases, were searched using the terms “laparoscopic partial nephrectomy”, “3D laparoscopic surgery”, “3D laparoscopy”, “3D laparoscopic partial nephrectomy”, “three-dimensional laparoscopic partial nephrectomy”, and “robotic partial nephrectomy” in May 2023. The searches were carried out independently by two reviewers.
All articles that included LPN surgeries performed using 3D laparoscopic systems (either as a single arm or in comparison with 2D laparoscopy or robotic system) that were published in the English language (articles with at least abstract available in the English language were also considered), randomized or observational studies, performed in adults or children were included. Studies that compared 3D laparoscopy with robotic surgeries in non-urological/non-renal cancer patients, commentaries, editorials, and letters to editors were excluded. The search strategy was given in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 18th May 2023 |
| Databases and other sources searched | PubMed, Emabse, Clinicaltrials.gov, Clinical key databases and grey literature, including Google Scholar databases |
| Search terms used | “laparoscopic partial nephrectomy”, “3D laparoscopic surgery”, “3D laparoscopy”, “3D laparoscopic partial nephrectomy”, “three-dimensional laparoscopic partial nephrectomy”, and “robotic partial nephrectomy” |
| Timeframe | 1990–2022 |
| Inclusion and exclusion criteria | All articles that included LPN surgeries performed using 3D laparoscopic systems (either as a single arm or in comparison with 2D laparoscopy or robotic system) that were published in the English language (articles with at least abstract available in the English language were also considered), randomized or observational studies, performed in adults or children were included. Studies that compared 3D laparoscopy with robotic surgeries in non-urological/non-renal cancer patients, commentaries, editorials, and letters to editors were excluded |
| Selection process | Conducted independently by Rohith G and Das MK; authors discussed the literature, results and obtained consensus |
| Any additional considerations, if applicable | References of the selected articles were also reviewed for relevant literature |
LPN, laparoscopic partial nephrectomy; 3D, three-dimensional; 2D, two-dimensional.
Results
The literature search generated nine studies describing 3D-LPN. There were no studies that had a direct comparison of 3D-LPN with RPN. Most of the studies were small comparative studies with 2D-LPN. Data pertaining to study design, tumour-related characteristics, intraoperative considerations such as warm ischemia time (WIT), positive surgical margins (PSMs), and change in the renal function following surgery, follow-up data were extracted from the select articles. The cumulative results are shown in Table 2.
Table 2
| Ref. | No. of arms | Study arms | No. of patients | Tumour characters (mean ± SD/median [range]) | WIT (mean ± SD/median [range]) | Positive margins | Compilations | Follow-up (median [range]/median) | Renal function (mean ± SD/median [range]) |
|---|---|---|---|---|---|---|---|---|---|
| Ruan, 2016 (15) | Two | 3D-LPNSSAC vs. 2D-LPN | 90 (45 vs. 45) | Size <7 cm | 22.6±4.2 vs. 19.7±4.4 min | 0 | Total incidence: 8.8%. Grade 1: (hematuria): 7 cases (3 vs. 4). One pseudoaneurysm embolization: 2D-LPN group | Follow-up time: 16.8 [5.5–22.5] months. No recurrences | Change in bilateral GFR: −8.5±7.2 vs. −7.9±6.4 mL/min; Change in ipsilateral GFR: −12.4±5.6 vs. −8.9±5.2 mL/min |
| Wang, 2015 (16) | Two | 3D retroperitoneal LPN with 3D-IDM created using 3D-MIRGS (with model reconstruction vs. without 3D reconstruction) | 35 (21 vs. 14) | Median tumor size: 2.9 [1.3–4.4] vs. 3.4 [1.4–4.2] cm; Median RNS: 7.0 [4–9] vs. 6.9 [5–8] | Mean selective renal artery clamp time: 28.1 [13–41] vs. 29.4 [21–35] min | 0 | Grade I (hematuria): 7 vs. 8; Grade II (transfusion): 9 vs. 10; Grade III (urinary leakage): 1 vs. 5 | 6 months | Absolute change in serum creatinine at 6 months follow-up: 9.1% vs. 1.9% |
| Komatsuda, 2016 (17) | Two | 3D-LPN vs. 2D-LPN | 31 (11 vs. 20) | Size: <4 cm (2.0±0.8 cm); RNS: 6.9±1.9 | 16.1 vs. 21.2 min, P=0.02 | – | 10% vs. 9%. No grade III or IV complications | 3 months | eGFR change at 3 months postop: −7.2±8.5 mL/min/kg; Between groups: −8.0±10.0 vs. −5.7±4.4 mL/min/kg |
| Tan, 2017 (18) | Two | 3D-LPN vs. 2D-LPN | 134 (53 vs. 81) | – | 23.70±6.96 vs. 26.60±8.10 min, P=0.032 | 0 | Comparable incidence of complications | 1–32 months. No recurrence, renal failure, metastasis or death | Decrease of GFR of the operated kidney: 12.70±6.49 vs. 15.10±6.45 mL/min/1.73 m2, P=0.036 |
| Hu, 2019 (19) | Two | 3D-LPN vs. 2D-LPN | 94 (47 vs. 47) | Padua score >10 | 27 [22–40] vs. 19 [15–28] min, P<0.001 | 1 (LPN group) | 14.9% vs. 23.4%; P<0.01. All grade I & II. One case in LPN required embolization | 18.5 months. One local recurrence in LPN | Change in GFR at 12 months: −8.7 [−9.5, −5.8] vs. 0 [−2, 3], P<0.01 |
| Introini, 2020 (20) | Single | Clamp less and suture less 3D laparoscopic partial nephrectomy | 62 | <4 cm, RNS: 4–6 | – | 2 | Grade I: 7 (11.2%); Grade II: 4 (6.4%); Grade III: 1 (1.6%) | No recurrence at 38.5-month median follow-up | Preop GFR: 92 [55–125]; Postop GFR at 3 months: 88 [55–120], P=0.09 |
| Dobrota, 2020 (21) | Two | 3D laparoscopic enucleation versus standard partial nephrectomy | 83 | <4 cm: 63 patients; ≥4 cm: 20 patients | – | – | – | 1 year | Preop GFR: 80.1±21.5 mL/min; Postop GFR: 75.3±22.4 mL/min |
| Li, 2020 (5) | Two | 3D-LPN vs. 2D-LPN | 76 (42 vs. 34) | RNS ≥10 | 22.5±6.8 vs. 28.7±7.8 min, P=0.0002 | 0 | 7.1% vs. 8.8%. No grade III or IV complications | 1–36 months | eGFR changes: 8.5±1.2 vs. 9.0±1.6 mL/min/1.73 m2, P=0.124 |
| Tokas, 2021 (22) | Two | 3D-LPN vs. 2D-LPN | 112 (52 vs. 60) | 3.00 [1.00–5.00] vs. 3.70 [1.50–6.40] cm | 11.50 [0–28] vs. 10.00 [0–25] min | – | Similar (P=0.55). Incidence not mentioned | – | Median eGFR (based on MDRD) decrease: 0 [−23.9, 126.3] vs. 0 [−29.2, 38.3] mL/min/1.73 m2 |
3D, three-dimensional; LPN, laparoscopic partial nephrectomy; WIT, warm ischemia time; 3D-LPNSSAC, 3D-LPN with selective segmental artery clamping; 2D, two-dimensional; GFR, glomerular filtration rate; 3D-IDM, 3D individual digital model; 3D-MIRGS, 3D medical image reconstructing and guiding system; RNS, RENAL nephrometry score; RENAL, radius, endophytic, nearness to collecting system, anterior/posterior, and location; eGFR, estimated GFR; MDRD, Modification of Diet in Renal Disease.
The advent of modern-day 3D laparoscopic systems
Technological advancements have revolutionized surgical video systems, enhancing image quality, comfort, and precision for surgeons. Initially, 3D monitors with low visual ergonomics and heavy active shutter glasses resulted in poor image quality. It was notorious for causing side effects like early fatigue, headaches, ocular fatigue, and sometimes nausea. However, recent developments have addressed these issues.
Dual-channel video technology connects a dual-channel optical scope to two cameras, displaying two images on a stereoscopic screen. Using polarized 3D glasses, a sense of depth can be perceived as the brain merges the images. The dual chip-on-the-tip technology bypassed optical distortions by mounting two video chips at the end of the scope, but due to the proximity of the chips, limited 3D effects are perceived (23).
The deflectable tip laparoscope provides flexibility for 100 degrees of tip rotation in four directions enabling the surgeons to maintain image orientation, obtain a critical clinical view, and improve depth perception and depth of field. Autostereoscopic displays eliminate the need for additional viewing devices by utilizing liquid crystal display (LCD) technology. The screen emits light at different angles, creating a parallax effect that allows surgeons to perceive depth without polarized glasses.
Benefits of 3D laparoscopy over 2D laparoscopy and robotic surgery
- Depth perception: 3D visualization enhances depth perception, allowing better judgment of distances and spatial relationships between anatomical structures. This helps in accurately assessing tissue depth and performing precise movements during surgery.
- Retained tactile feedback: 3D laparoscopy allows surgeons to maintain tactile feedback, unlike robotic-assisted surgeries. By directly feeling tissues’ resistance, texture, and consistency, performing delicate manoeuvres and identifying critical structures becomes more feasible.
- Accuracy and safety: The enhanced depth perception and preserved tactile feedback contribute to increased surgical accuracy. Tissues can be better differentiated, and precise dissections, suturing, and tissue manipulation can be safely performed. This along with enhanced visualization will increase the safety.
- Reduced strain: studies have shown a reduced strain on the operating surgeons (24).
- Surgical precision: with better visualization and improved accuracy, 3D laparoscopy helps to perform complex procedures with greater precision. This can lead to better surgical outcomes and potentially reduce the need for additional interventions.
- Improved hand-eye coordination: the 3D visualization assists in better correlating the hand movements with the added visual information, allowing for more precise instrument manipulation.
- Low capital expenditure and low maintaining recurring costs: 3D laparoscopy typically involves lower initial capital expenditure than robotic surgical systems. It can be integrated into existing laparoscopic setups with minimal additional equipment requirements. They often utilize conventional instruments, which reduces the need for specialized and costly robotic instruments (23).
- Conventional and new straight stick instruments/articulating instruments: surgeons can use traditional laparoscopic and newer straight stick instruments with 3D laparoscopy. This flexibility allows surgeons to leverage their existing instrument inventory while utilizing improved visualization. Articulating instruments have made their way into the present-day laparoscopy armamentarium, which can be safely integrated with 3D systems (25).
- Shorter learning curve: the transition from 2D to 3D laparoscopy typically has a shorter learning curve than robotic surgery. Surgeons familiar with conventional laparoscopy can adapt more quickly to 3D visualization and continue to build on their existing skills (26).
Learning curve: how is it influenced?
LPN demands considerable levels of skills as well as expertise when compared to other urological procedures. Based on the available literature, even with laparoscopic prior knowledge, the learning curve for LPN stands at around 100 to 150 procedures concerning operating time (27). However, the number was calculated in the mid-2000 and significant inventions and adjuncts were added to laparoscopic surgery that could considerably reduce the above-stated number. In a study conducted by Haseebuddin et al., he stated that the learning curve for RPN was only 26 cases when a fellowship-trained laparoscopic surgeon was evaluated for his learning curve (28).
Intracorporal suturing is one of the most significant determining factors that influence the operative duration. The surgeon often performs the procedure under the duress of WIT. Hubens et al. conducted a study on the efficacy of intracorporeal suturing using robotic systems, 2D and 3D laparoscopy systems. Twenty surgeons were recruited and divided into experts (over 100 laparoscopic cases), surgeons with intermediate experience (20–99 cases), and novices (less than 20 cases). Each participant was made to perform an intracorporeal suturing task three times, and task failure rates and completion times were recorded. All novices could not complete the task using 2D or 3D laparoscopy, but they were successful with the robotic system. The intermediate group had a higher failure rate with 2D laparoscopy (23.8%) compared to 3D laparoscopy (4.8%) and the robot (0%). The completion times for the intermediate group were similar across all three instruments. Among the experts, the failure rates were low for all instruments, but their completion times were significantly faster with 3D laparoscopy compared to 2D laparoscopy. The experts’ completion times with 3D laparoscopy were shorter than the robotic system. Novice surgeons showed notable advantages when using the robotic system, while the intermediate group achieved similar performance and speed with both 3D laparoscopy and the robot. Experts demonstrated proficiency regardless of the modality but completed the task significantly faster with 3D laparoscopy. These results imply that highly skilled laparoscopic surgeons may perform tasks with 3D laparoscopic systems equally proficiently as robotic surgical systems (29).
Advanced techniques such as preoperative planning and real time navigation can be obtained using 3D laparoscopy systems. In a study on 44 cT1 renal tumor patients 3D-LPN using individual digital models (3D-IDM) and real-time navigation were evaluated and the 3D-medical image reconstructing and guiding system (MIRGS) group (n=21) demonstrated shorter operative time (159.0 vs. 193.2 min; P<0.001) and reduced blood loss (148.1 vs. 176.1 mL; P<0.001) compared to controls (n=14) (16).
The complexity of renal masses
Most of the studies on 3D-LPN included T1 tumours (less than 7 cm) (Table 2). Radius, endophytic, nearness to collecting system, anterior/posterior, and location (RENAL) nephrometry scores (RNSs) were less, along with the sizes of the tumours included in the available studies before the 2020s. Li et al. and Hu et al. compared the performance of the 3D-LPN with 2D-LPN in tumours with RNS ≥10 and Padua scores >10, respectively (5,19). Another prospective comparative study between 3D-LPN and 2D-LPN also showed similar results with reduced WIT, blood loss and operative durations with 3D laparoscopy (22). Both studies reported a significantly reduced WIT with 3D visualization systems. Even for smaller and less complex tumours, the outcome parameters were relatively comparable except for a significant reduction in the WIT (17,18).
Outcomes of 2D-LPN were compared with RPN extensively, and the results showed similar outcomes concerning operative duration, PSM rates, and surgical complications. In a meta-analysis performed in small renal masses in 2013 in 256 patients, operative time, blood loss, and postoperative complications were comparable with both LPN and RPN (3). Similarly, another study was performed with a large patient population of 717 patients, and both LPN and RPN had comparable perioperative outcomes except for longer WIT with LPN (30). In 2,240 patients who underwent PN using either modality, significantly better outcomes were reported concerning conversion to open surgery or RN, WIT, change in estimated glomerular filtration rate (eGFR), and LOH were less in patients who underwent RPN (31).
The real expansion of the tumour complexity boundary was achieved with the introduction of RPN, where the intra-corporeal suturing was performed with greater ease. Seven-degree-of freedom achieved with articulating instruments, tremor dampening, superior image quality, and 3D vision paved the way for better overall outcomes RPN (8). However, most of the studies available were performed with the study groups comparing 2D-LPN with 3D-LPN. We could already elicit the superiority of 3D-LPN over 2D-LPN with smaller studies. So, putting the data together, we can assume that the outcomes with 3D-LPN fare better than 2D-LPN but can be inferior to RPN owing to other apparent advantages of surgical robots. A well-designed randomized control trial comparing the three modalities is the need of the hour to better define the differences in the outcomes with respect to treating complex renal masses.
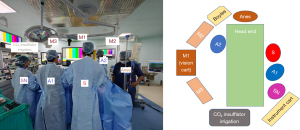
In our experience, we transitioned from a 2D video system to a 3D video system 5 years ago. Initially, we had limited ourselves to T1a renal masses. Following the acquisition, we performed more complex tumours such as hilar, T1b and T2 renal masses without significantly increasing WIT or postoperative complications. Nearly 114 PN surgeries have been performed in our institute, with 74 LPNs performed with the 3D video system. The schemata of operating theatre along with patient 3D setup is depicted in Figure 1.
Minimizing renal ischemia
Performing off-clamp PN (OC PN) or selective arterial clamping, along with various technological advances in imaging systems, paved the way for improving the WIT duration during PN. In 2016, Ruan and his colleagues performed selective segmental arterial clamping during 3D-LPN and compared the outcomes with 2D-LPN in T1 renal masses. Although the technique was noted to have higher blood loss (P<0.01), there was a significant reduction in the WIT (P=0.04) and a significantly better ipsilateral renal function (P<0.01) (15). Recently, Introini reported the outcomes of 62 patients with small renal masses (T1a) who underwent clamp-less and suture-less 3D-LPN. Although two patients had focal surgical margin positive, no recurrence was noted at a median follow-up duration of 38.5 months and no significant difference in the GFR was noted at a 3-month follow-up (20).
OC techniques in RPN had reported mixed results. OC RPN reported similar eGFR rates in both OC RPN and on-clamp RPN groups at 9 months follow-up (32). One of the recent systematic reviews and meta-analyses on 4,493 patients who underwent RPN using OC and on clamp, RPN reported a better postoperative renal functional outcome, shorter operative duration, and lower complications in patients who underwent OC RPN, despite higher intraoperative blood loss (33). But due to heterogeneity in the studies considered for the study and potential bias, the results of this meta-analysis may hinder its application to larger patient populations.
Long-term functional outcomes of OC PN were described by Shah and colleagues in 2016 in 315 patients who underwent LPN which stated that the functional benefit that is seen at the usual 6 months postoperative period does not translate into a long-term use which is evident by the comparable renal function and similar incidence of chronic kidney disease in the long-term. So, the functional benefit from eliminating the transient warm ischemia with increased blood loss may not be of any clinical benefit in the long run (34).
A recent systematic review of eight studies compared standard RPN with LPN and open partial nephrectomy (OPN). Ruiz Guerrero and his colleagues stated there was an overall decline in the WIT with the robotic approach compared to others. The range observed in robotic surgery was between 18 and 24.7 minutes, with the higher end more common in larger and more complex tumours exceeding 2 cm in size. In LPN, the range for WIT was between 21 and 24 minutes. However, the data summarized is only for two randomized studies, whereas the other studies were very heterogeneous (35).
PSMs
Amongst the available literature on 3D-LPN, the incidence of PSM is less. Although the progression clinical significance of the PSM leading to clinically significant local recurrence and progression to metastasis along with a reduction in the survival rates were not seen in many previous studies. The impact of a particular surgical approach on the margin status has largely been inconclusive.
In a large multi-institutional study, the three available modalities, open, laparoscopic, and robotic PN, were compared in 285 patients with T1b renal masses. Surprisingly, OPN demonstrated higher PSM (6.8%) when compared with LPN (1.9%) and RAPN (2.5%), although the difference is statistically insignificant. A large database-based analysis of 11,587 patients who had undergone PN for T1a renal masses reported PSMs in around 7% of the cases. The adjusted odds ratio was 1.81 for LPN and 1.79 for RPN when compared with OPN (36).
The incidence of PSM in PN surgery classically was around 2–8% (36). The clinical significance of PSM is unclear mainly because of the varied incidence in the articles that report the data and inherent bias due to the predominantly retrospective nature of the articles that described them. There appears to be no influence on the survival outcomes related to the PSM status in most of them (37,38). However, there was an increase in the local recurrence and metastasis rates in a few studies (39). The incidence of local recurrences at the tumour bed stands at 16% in patients who had PSM when compared with 3% recurrences that were reported in patients with negative margins (40).
Postoperative complications
Apart from two studies in which the patients had to undergo embolization of postoperative bleeding or pseudoaneurysm development, all the patients in the stated articles on 3D-LPN had only grade I or II complications without any higher-grade complications. The overall incidence in most studies is under 10%, and the rates are comparable to 2D-LPN groups (5,15,17). Except for one study by Hu and his colleagues that was performed in 2019, a statistically significant reduction in postoperative complications was noted in patients who underwent 3D-LPN (19).
In all the studies, the major and minor complications were graded using Clavian-Dindo classification. The incidence rates of these complications were similar to the available rates previously described in studies comparing LPN, OPN and RPN procedures (41). Various studies that described these intraoperative and postoperative complications were known to be heterogenous concerning patient population characteristics, sample size, and interventions described. Porpiglia compared 285 patients with the three modalities in the RECORd project, which demonstrated a higher complication rate with OPN (12.8%) when compared with LPN (1.8%) and RPN (2.1%) (7). Also, the mode of resection significantly impacts PN outcomes. Dobrota et al.’s study, utilizing 3D laparoscopy, revealed a decrease in GFR from 80.1±21.5 to 75.3±22.4 mL/min. Standard PN resulted in a more substantial reduction in GFR compared to enucleation (21). Although the incidence of postoperative complications forms a part of the margin, ischemia, complication (MIC) score, which is frequently used to compare modalities of surgical approach to perform PN, significant differences in the postoperative outcomes were seen when comparing open versus minimally invasive techniques. Whereas in most of the available studies, the incidence of the complications was mainly of the lower grade and comparable in patients undergoing minimally invasive PN either via laparoscopic approach or a robotic approach.
Costs
With the paradigm shift seen with the introduction of minimally invasive techniques in the early 1990s, there was an ever-increasing trend of adopting novel surgical modalities, especially in urology. Recent years have seen a surge in the adoption of robotic surgery in performing complex urological procedures, especially in the Western population. Despite robotic surgical systems posing indubitable advantages, it is to be taken into consideration the economic burden associated with running a successful robotic surgical platform when compared with conventional minimally invasive surgical procedures such as laparoscopy.
Despite boasting various favourable clinical outcomes, robotic surgical systems failed to gain worldwide extensive adaptation because of their high costs (42). Compared with laparoscopy, the costs are not only limited to acquiring the robotic system, but also for its regular maintenance charges and high costs associated with the consumables. The price for acquiring a standard robotic system ranges anywhere between 2 million dollars to 2.5 million dollars. As a surplus, the annual service charge alone costs around 200,000 dollars (43). A meta-analysis comparing OPN, LPN, and RPN was conducted to assess the direct costs incurred in performing the procedures. The analysis included data from multiple studies, totalling 477 RPN procedures, 2,220 LPN procedures, and 2,745 OPN procedures. The weighted mean operating room (OR) times were similar for all three approaches, ranging from 188 to 200 minutes. However, LPN had the shortest LOH at 2.6 days, followed by RPN at 3.2 days, and the OPN had a LOH of 5.9 days. Based on cost models using institution-specific data, LPN was the most cost-effective approach, with a mean direct cost of $10,311. It had a cost advantage of $1,116 over OPN ($11,427) and $1,652 over robot assisted laparoscopic partial nephrectomy (RALPN) ($11,962) (42). LPN’s cost-effectiveness was primarily attributed to its shorter LOS, despite having higher instrumentation costs than RALPN. Sensitivity analyses revealed that significant reductions in robotic costs were necessary for RALPN to become cost-effective.
Limitations
The narrative nature of the present review stands as a limitation as there would be a chance for missing information due to missing studies. Also, there does not exist robust level one evidence that compares 3D-LPN with RPN and thus, the inferences deduced were mostly a correlation between previous comparative studies (2D-LPN and 3D-LPN) and the newer studies on RPN. Also, the lack of headon studies precluded the objective analysis of the outcomes that were discussed.
Thus, with this review we would like to state that there exists an imperative need for large randomized trials comparing both modalities to develop robust evidence for the effectiveness of minimally invasive approaches in the treatment of renal cancer.
Conclusions
With obvious advantages over the open approach and less costs associated, laparoscopic approach stands as a more cost-effective alternative for robotic surgery platforms in performing PN surgery. However, with an unquestionable advantage over other approaches in terms of less WIT, robotic surgery is gaining its importance at a noticeable pace for performing complex urological surgeries.
Laparoscopy augmented with 3D vision remains a plausible added advantage to the already efficient traditional laparoscopic system making it more acceptable, and cost-effective alternative to robotic surgery. It also shortens the learning curve of novice surgeons and augments their laparoscopic skill-sets. In surgeons with experienced hands, PN can be successfully performed far more safely, efficiently, and cost-efficiently using 3D-laparoscopy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Nitin Sharma) for the series “Laparoscopic Urology” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-23-38/rc
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-38/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-38/coif). The series “Laparoscopic Urology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vartolomei MD, Matei DV, Renne G, et al. Robot-assisted Partial Nephrectomy: 5-yr Oncological Outcomes at a Single European Tertiary Cancer Center. Eur Urol Focus 2019;5:636-41. [Crossref] [PubMed]
- Das MK, Rohith G, Mandal S, et al. Intraoperative ultrasonography (IOUS)-guided vs conventional laparoscopic nephrectomy: a randomized control trial. BJU Int 2024;133:71-8. [Crossref] [PubMed]
- Froghi S, Ahmed K, Khan MS, et al. Evaluation of robotic and laparoscopic partial nephrectomy for small renal tumours (T1a). BJU Int 2013;112:E322-33. [Crossref] [PubMed]
- Ng AM, Shah PH, Kavoussi LR. Laparoscopic Partial Nephrectomy: A Narrative Review and Comparison with Open and Robotic Partial Nephrectomy. J Endourol 2017;31:976-84. [Crossref] [PubMed]
- Li M, Ren Y, Weng G. Clinical Study of Three-Dimensional Laparoscopic Partial Nephrectomy for the Treatment of Highly Complex Renal Tumors with RENAL Nephrometry Scores of ≥10 Points. Biomed Res Int 2020;2020:5824209. [Crossref] [PubMed]
- Pavan N, Derweesh IH, Mir CM, et al. Outcomes of Laparoscopic and Robotic Partial Nephrectomy for Large (>4 Cm) Kidney Tumors: Systematic Review and Meta-Analysis. Ann Surg Oncol 2017;24:2420-8. [Crossref] [PubMed]
- Porpiglia F, Mari A, Bertolo R, et al. Partial Nephrectomy in Clinical T1b Renal Tumors: Multicenter Comparative Study of Open, Laparoscopic and Robot-assisted Approach (the RECORd Project). Urology 2016;89:45-51. [Crossref] [PubMed]
- Thiel DD, Winfield HN. Robotics in urology: past, present, and future. J Endourol 2008;22:825-30. [Crossref] [PubMed]
- Dai X, Fan S, Hao H, et al. Comparison of KD-SR-01 robotic partial nephrectomy and 3D-laparoscopic partial nephrectomy from an operative and ergonomic perspective: A prospective randomized controlled study in porcine models. Int J Med Robot 2021;17:e2187. [Crossref] [PubMed]
- Li X, Xu W, Fan S, et al. Robot-assisted Partial Nephrectomy with the Newly Developed KangDuo Surgical Robot Versus the da Vinci Si Surgical System: A Double-center Prospective Randomized Controlled Noninferiority Trial. Eur Urol Focus 2023;9:133-40. [Crossref] [PubMed]
- Sheetz KH, Claflin J, Dimick JB. Trends in the Adoption of Robotic Surgery for Common Surgical Procedures. JAMA Netw Open 2020;3:e1918911. [Crossref] [PubMed]
- Petrut B, Bujoreanu CE, Porav Hodade D, et al. Indocyanine green use in Urology. J BUON 2021;26:266-74. [PubMed]
- Lusch A, Bucur PL, Menhadji AD, et al. Evaluation of the impact of three-dimensional vision on laparoscopic performance. J Endourol 2014;28:261-6. [Crossref] [PubMed]
- Cicione A, Autorino R, Breda A, et al. Three-dimensional vs standard laparoscopy: comparative assessment using a validated program for laparoscopic urologic skills. Urology 2013;82:1444-50. [Crossref] [PubMed]
- Ruan Y, Wang XH, Wang K, et al. Clinical evaluation and technical features of three-dimensional laparoscopic partial nephrectomy with selective segmental artery clamping. World J Urol 2016;34:679-85. [Crossref] [PubMed]
- Wang D, Zhang B, Yuan X, et al. Preoperative planning and real-time assisted navigation by three-dimensional individual digital model in partial nephrectomy with three-dimensional laparoscopic system. Int J Comput Assist Radiol Surg 2015;10:1461-8. [Crossref] [PubMed]
- Komatsuda A, Matsumoto K, Miyajima A, et al. Technical Improvement Using a Three-Dimensional Video System for Laparoscopic Partial Nephrectomy. Asian Pac J Cancer Prev 2016;17:2475-8. [PubMed]
- Tan HS, Tang XH, Wu ZJ, et al. Clinical comparative analysis of 3D and 2D laparoscopic partial nephrectomy for renal tumors. Academic Journal of Naval Medical University 2017;38:239-43.
- Hu M, Guan C, Xu H, et al. Comparison of 3-dimensional laparoscopy and conventional laparoscopy in the treatment of complex renal tumor with partial nephrectomy: A propensity score-matching analysis. Medicine (Baltimore) 2019;98:e17435. [Crossref] [PubMed]
- Introini C, Di Domenico A, Ennas M, et al. Functional and oncological outcomes of 3D clampless sutureless laparoscopic partial nephrectomy for renal tumors with low nephrometry score. Minerva Urol Nefrol 2020;72:723-8. [Crossref] [PubMed]
- Dobrota F, Andras I, Gherle B, et al. 3D laparoscopic enucleation vs standard partial nephrectomy for Ct1 renal masses: assessment of functional outcomes at 1-year follow-up. Ann Ital Chir 2020;91:321-6. [PubMed]
- Tokas T, Avgeris M, Leotsakos I, et al. Impact of three-dimensional vision in laparoscopic partial nephrectomy for renal tumors. Turk J Urol 2021;47:144-50. [Crossref] [PubMed]
- Sinha RY, Raje SR, Rao GA. Three-dimensional laparoscopy: Principles and practice. J Minim Access Surg 2017;13:165-9. [Crossref] [PubMed]
- Currò G, La Malfa G, Lazzara S, et al. Three-Dimensional Versus Two-Dimensional Laparoscopic Cholecystectomy: Is Surgeon Experience Relevant? J Laparoendosc Adv Surg Tech A 2015;25:566-70. [Crossref] [PubMed]
- Kim JK, Kang B, Kim YS, et al. Laparoscopic renal surgery using multi degree-of-freedom articulating laparoscopic instruments in a porcine model. Investig Clin Urol 2023;64:91-101. [Crossref] [PubMed]
- Blavier A, Gaudissart Q, Cadière GB, et al. Comparison of learning curves and skill transfer between classical and robotic laparoscopy according to the viewing conditions: implications for training. Am J Surg 2007;194:115-21. [Crossref] [PubMed]
- Link RE, Bhayani SB, Allaf ME, et al. Exploring the learning curve, pathological outcomes and perioperative morbidity of laparoscopic partial nephrectomy performed for renal mass. J Urol 2005;173:1690-4. [Crossref] [PubMed]
- Haseebuddin M, Benway BM, Cabello JM, et al. Robot-assisted partial nephrectomy: evaluation of learning curve for an experienced renal surgeon. J Endourol 2010;24:57-61. [Crossref] [PubMed]
- Hubens G, Coveliers H, Balliu L, et al. A performance study comparing manual and robotically assisted laparoscopic surgery using the da Vinci system. Surg Endosc 2003;17:1595-9. [Crossref] [PubMed]
- Aboumarzouk OM, Stein RJ, Eyraud R, et al. Robotic versus laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol 2012;62:1023-33. [Crossref] [PubMed]
- Choi JE, You JH, Kim DK, et al. Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol 2015;67:891-901. [Crossref] [PubMed]
- Anderson BG, Potretzke AM, Du K, et al. Off-clamp robot-assisted partial nephrectomy does not benefit short-term renal function: a matched cohort analysis. J Robot Surg 2018;12:401-7. [Crossref] [PubMed]
- Huang Y, Cao D, Chen Z, et al. Comparison of Perioperative, Renal Functional, and Oncological Outcomes Between Off-Clamp and On-Clamp Robot-Assisted Partial Nephrectomy for Renal Tumors: An Updated Evidence-Based Analysis. Front Oncol 2021;11:730662. [Crossref] [PubMed]
- Shah PH, George AK, Moreira DM, et al. To clamp or not to clamp? Long-term functional outcomes for elective off-clamp laparoscopic partial nephrectomy. BJU Int 2016;117:293-9. [Crossref] [PubMed]
- Ruiz Guerrero E, Claro AVO, Ledo Cepero MJ, et al. Robotic versus Laparoscopic Partial Nephrectomy in the New Era: Systematic Review. Cancers (Basel) 2023;15:1793. [Crossref] [PubMed]
- Tabayoyong W, Abouassaly R, Kiechle JE, et al. Variation in Surgical Margin Status by Surgical Approach among Patients Undergoing Partial Nephrectomy for Small Renal Masses. J Urol 2015;194:1548-53. [Crossref] [PubMed]
- Antic T, Taxy JB. Partial nephrectomy for renal tumors: lack of correlation between margin status and local recurrence. Am J Clin Pathol 2015;143:645-51. [Crossref] [PubMed]
- Petros FG, Metcalfe MJ, Yu KJ, et al. Oncologic outcomes of patients with positive surgical margin after partial nephrectomy: a 25-year single institution experience. World J Urol 2018;36:1093-101. [Crossref] [PubMed]
- Carvalho JAM, Nunes P, Tavares-da-Silva E, et al. Impact of Positive Surgical Margins After Partial Nephrectomy. Eur Urol Open Sci 2020;21:41-6. [Crossref] [PubMed]
- Wood EL, Adibi M, Qiao W, et al. Local Tumor Bed Recurrence Following Partial Nephrectomy in Patients with Small Renal Masses. J Urol 2018;199:393-400. [Crossref] [PubMed]
- Ricciardulli S, Ding Q, Zhang X, et al. Evaluation of laparoscopic vs robotic partial nephrectomy using the margin, ischemia and complications score system: a retrospective single center analysis. Arch Ital Urol Androl 2015;87:49-55. [Crossref] [PubMed]
- Mir SA, Cadeddu JA, Sleeper JP, et al. Cost comparison of robotic, laparoscopic, and open partial nephrectomy. J Endourol 2011;25:447-53. [Crossref] [PubMed]
- Barbash GI, Glied SA. New technology and health care costs—the case of robot-assisted surgery. N Engl J Med 2010;363:701-4. [Crossref] [PubMed]
Cite this article as: Rohith G, Das MK. Kidney cancer: 3D laparoscopic partial nephrectomy—role in the current era of robotic surgery—a narrative review. Ann Laparosc Endosc Surg 2024;9:4.


